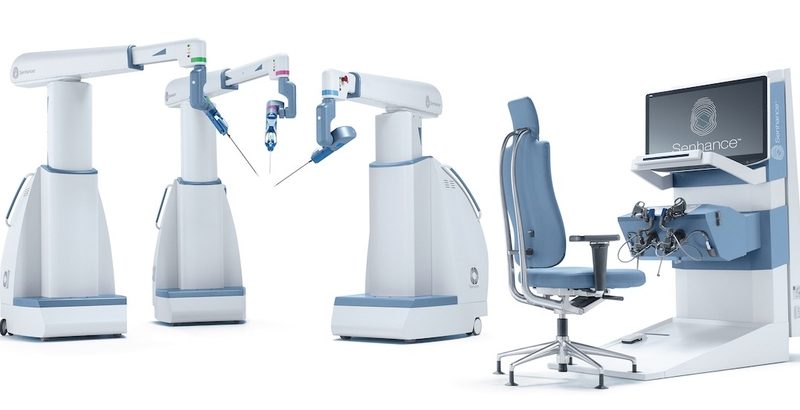
TransEnterix received an FDA clearance for its “digital surgical assistant,” which it describes as the first machine vision system for robotic surgery.
The company’s Intelligent Surgical Unit is designed as an add-on to its laparoscopic Senhance system, to help augment control of the surgeon’s camera during a minimally invasive procedure. It was first submitted to the FDA for review in mid-January.
“We are pleased to have received this important clearance earlier than expected,” said TransEnterix President and CEO Anthony Fernando in a statement. “Machine vision is the next major advance in digital surgery.”
By digitally recognizing certain objects, organs and locations within the field of view—along with eye-tracking features, operator commands and learning certain preferences over time—the AI system is designed to anticipate what the surgeon wants to see during a procedure and adjusts the focus and zoom of the camera accordingly.
“Our system is designed to significantly advance the sensing capabilities of computer-assisted surgery,” Fernando said. “With this hardware and software system, the Senhance System will gather and interpret visual information from the surgical field. The capabilities now cleared will be focused on optimizing visualization and camera control in ways never before offered in robotic or digital surgery.”
Fernando described the clearance of these capabilities as the first step in the company’s efforts to bring more augmented intelligence and machine vision features to robotic surgery. This includes plans to develop advanced scene cognition and surgical image analytics in digital laparoscopy.