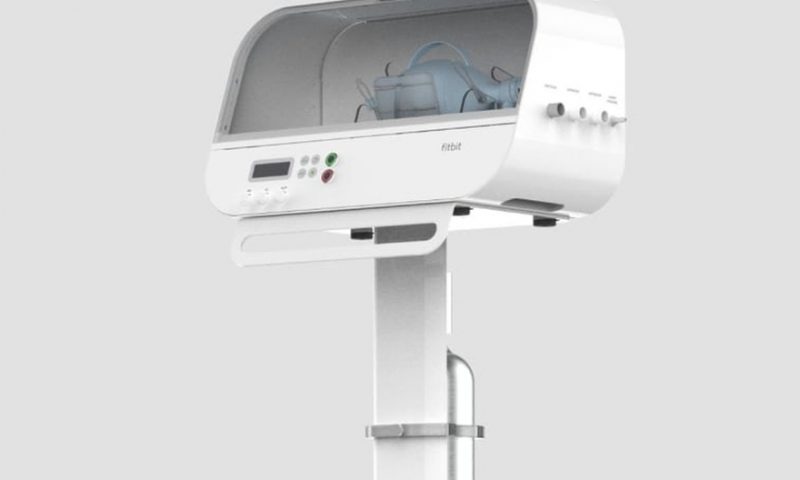
The FDA granted new COVID-19 green lights to Fitbit’s emergency ventilator alternative, as well as to a second device designed by engineers at NASA’s Jet Propulsion Laboratory.
Fitbit’s Flow ventilator is based on an inherently simple design seen as a response to the nationwide shortages of high-end hospital ventilator equipment—using mechanical arms to regularly squeeze a common bag-valve mask, used in ambulances and emergency rooms, to push oxygen into the lungs.
But Fitbit’s device takes the concept a little further, with options to control air delivery by volume or pressure, and includes an “assist control” feature that supports breaths triggered by the patient, according to the FDA.
Designed for adult COVID-19 patients, the machine acts as an accessory to equipment previously approved by the FDA, including manual, bag-valve resuscitators and single-use, disposable air tubing. The agency said the Flow device is only to be used when a clinical ventilator is unavailable due to the pandemic.
Fitbit CEO James Park, in a previous interview with CNBC, said the company’s device would be more advanced compared to emergency alternatives that simply provide breaths at a fixed rate per minute, but would still come in at a lower price point compared to the more durable hospital ventilators—machines designed to run constantly with finer controls, while adding heat and humidity to the air.
Developed in collaboration with physicians at Oregon Health & Science University, Massachusetts General Hospital and Brigham and Women’s Hospital, Park said production efforts could be based through its existing vendors in Taiwan.
“COVID-19 has challenged all of us to push the boundaries of innovation and creativity, and use everything at our disposal to more rapidly develop products that support patients and the healthcare systems caring for them,” Park later said in a company blog post. “We saw an opportunity to rally our expertise in advanced sensor development, manufacturing, and our global supply chain to address the critical and ongoing need for emergency ventilators and help make a difference in the fight against this global virus.”
Meanwhile, the FDA gave an emergency authorization to a second ventilator from NASA. The space agency’s previous VITAL device, for Ventilator Intervention Technology Accessible Locally, was OK’d by the agency in early May.
That machine, a short-term solution designed to last three to four months, relied on a hospital’s building-wide gas supply, piped into each room to provide pressure for its operations. The newer ventilator includes an internal compressor to power its needs.
Both were designed to be assembled from components outside the traditional medical device supply chain, to avoid impacting the manufacturing of current hospital ventilators.