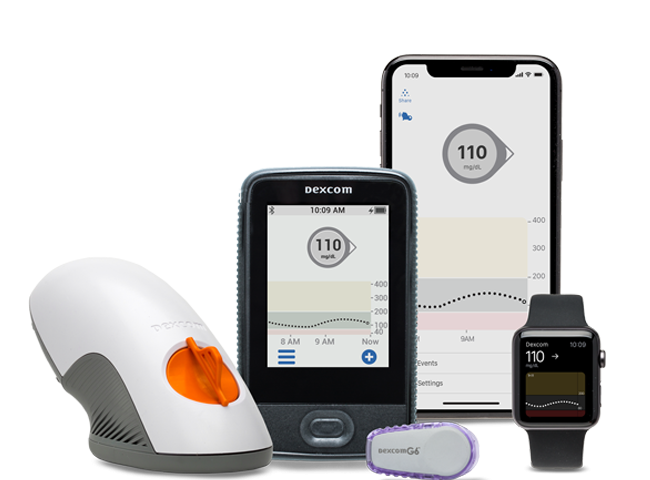
Dexcom has received approval in Europe extending the use of its wearable continuous glucose monitor (CGM) to pregnant women across Type 1, Type 2 and gestational diabetes.
Blood sugar levels can be difficult to manage during pregnancy, with insulin being pulled in two directions by both an increasing need and increasing resistance, the company said. Instead of multiple fingersticks per day and overnight, the G6 CGM system can provide regular tracking data through a smartphone app as well as alerts to help avoid potentially serious complications.
Announced during the International Conference on Advanced Technologies & Treatments for Diabetes in Madrid, Dexcom said the system will first be available in the U.K. this spring. The system had previously received CE mark approval for people age two and older.
Abbott’s competing FreeStyle Libre CGM system previously received European approval for pregnant women in 2017. No such system has been approved in the U.S. by the FDA, though many pregnant women with diabetes currently use them off-label.
“We are excited that the Dexcom G6 has received the CE mark in the E.U. for use during pregnancy,” said Erik Bjorkman, Dexcom’s general manager and vice president for the EMEA region. “It is a life-changing development for this group of patients and highlights our continued mission to innovate on behalf of everyone living with diabetes.”
Earlier last week, Dexcom said it would connect its current and future CGM systems with Insulet’s upcoming Omnipod Horizon tubeless insulin pump. Its glucose sensors will power Insulet’s predictive algorithm for determining insulin doses without the need for connecting additional devices.
The latest commercialization agreement builds upon Dexcom and Insulet’s previous tech integration work and formalizes plans to launch the Omnipod Horizon in the second half of this year.