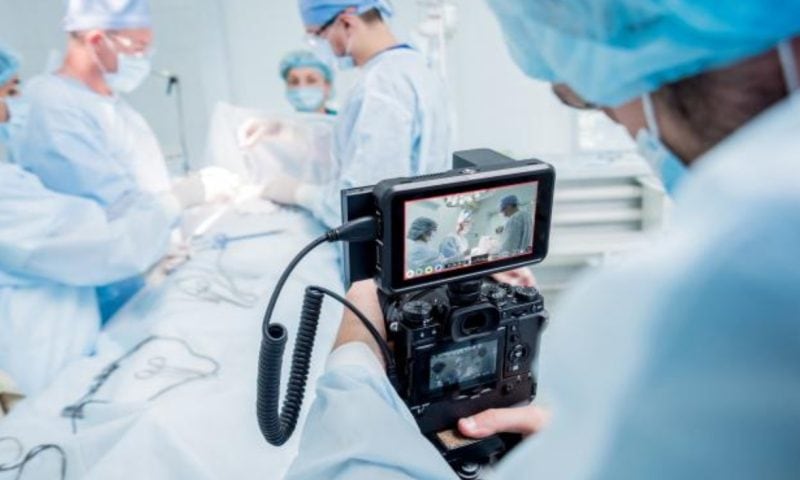
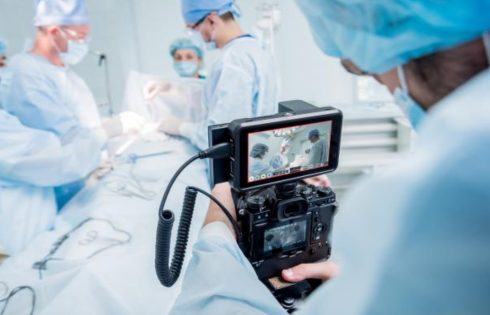
President Donald Trump’s exemption of pharmaceuticals from the “reciprocal” tariffs he announced Wednesday limited the declines of biopharma stocks as financial markets tanked late last week—but the specter of future industry-specific tariffs and import duties still hanging over the industry means that shares of biotech and pharma drug developers are hardly out of the proverbial woods.
Biopharma stocks mostly showed declines this past week, buffeted first by the sudden departure of Peter Marks, MD, as director of the FDA’s Center for Biologics Evaluation and Research (CBER), which pushed shares mostly downward on March 31—especially developers of cell therapies, gene therapies, other biologics, and vaccines, all overseen by CBER. The following day, the U.S. Department of Health and Human Services (HHS) began a staggering shrinkage of its workforce, eliminating some 10,000 jobs at the FDA, NIH, and U.S. Centers for Disease Control and Prevention (CDC).
A day later, Trump effectively ended the age of “free trade” by announcing a 10% minimum baseline tariff rate on all imports from all countries effective Saturday—plus reciprocal tariffs set at individual rates for more than 180 countries and territories with which the United States has run the largest trade deficits, including China (34% added to 20% tariff imposed in February), and the European Union (20%). The reciprocal tariffs take effect on April 9.
According to investment research and management firm Morningstar, last year the United States exported $95 billion in pharmaceutical products—but imported more than double that amount, about $200 billion.
“With roughly $200 billion in pharmaceutical imports in 2024, a 10% tariff could amount to a $20 billion headwind across the industry, with the biggest firms seeing potential annual tariffs as high as $1 billion,” predicted Karen Andersen, equity director with Morningstar, in a research note Thursday.
Figuring out which biopharmas face the greatest impact from future tariffs is a challenge, she added.
“While we have some information on which major products are manufactured in which countries, they are often produced in multiple geographies, making it difficult to determine the exact percentages being produced in the United States and whether finishing might be done in another country,” Anderson wrote.
While a future global pharmaceutical tariff could potentially lower gross margins while raising long-term tax rates, Andersen added: “We expect firms to be able to adapt their manufacturing, and nearly all large-cap biopharma firms continue to hold wide economic moats.”
Manufacturing plans “could help offset” tariff risks
Richard de Chazal, macroeconomic analyst with William Blair, noted in a report Thursday that several biopharma giants have announced plans for new U.S. manufacturing facilities over the past 12 months—including Amgen ($1 billion second drug substance manufacturing plant in Holly Springs, NC); Eli Lilly (four sites totaling $27 billion); Novo Nordisk ($4.1 billion second fill and finishing facility in Clayton, NC); and Johnson & Johnson (four plants totaling $55 billion-plus). Those plans, according to de Chazal, signal “a continued goal of globalized infrastructure that could help offset longer-term tariff risks.”
However, de Chazal cautioned that an earlier Trump threat of biopharma tariffs potentially 25% or higher “suggests the industry’s relief will be short-lived.
“If—or perhaps more appropriately, when—pharma-specific tariffs are rolled out, there will not be a material direct impact on most pharmaceutical outsourcing and services companies,” de Chazal observed. “That said, since tariffs would pressure margins for commercial drugs and likely make developing drugs more expensive, the taxes could potentially discourage investments in research and development, which in turn would lead to a more challenging demand environment for our coverage list.”
He said the brunt of any future tariff impacts on biopharma would likely be felt by pharma giants—“but it would also add to a large pile of unsettling news creating uncertainty around biotech funding and ultimately demand from smaller innovators.”
Among contract research organizations (CROs) and providers of tech-enabled services, de Chazal added, Charles River Laboratories (NYSE: CRL) would have the most direct exposure to tariff impacts, since about 18% of revenue is tied to non-pharmaceutical product sales, while Lonza Group (SIX Swiss Exchange: LONN) would also be negatively impacted by a slowdown in R&D spending, but to a lesser extent than other companies tracked by William Blair, since Lonza generates 70% of its contract development and manufacturing organization (CDMO) revenue from Phase III and commercial drugs. Lonza also has some potential direct exposure to tariffs through its capsules and health ingredients (CHI) segment, which accounted for 16% of 2024 revenue, the analyst noted.
“On the bright side, we believe Lonza is extremely well positioned to benefit from increased demand for U.S. manufacturing capacity” given its its $1.2 billion acquisition of Genentech’s large-scale biologics manufacturing site in Vacaville, CA, from parent company Roche last year, de Chazal added.
“Liberation” and engagement
During his “Liberation Day” ceremony Wednesday announcing the baseline and reciprocal tariffs, Trump didn’t mention his administration’s earlier threat of industry-specific tariffs. But he lamented what he called a dearth of U.S. drug making and predicted increased domestic manufacturing activity for biopharma would result from his policies.
“The United States can no longer produce enough antibiotics to treat our sick,” Trump said, adding: “We’re going to produce the cars, ships, chips, airplanes, minerals, and medicines that we need right here in America.”
“The pharmaceutical companies are going to come roaring back. They are coming roaring back. They are all coming back to our country because if they don’t, they got a big tax to pay. And if they do, I’ll be very happy,” Trump added.
Trump’s administration has specified the composition of pharmaceuticals exempted from the reciprocal tariffs in an annex to the executive order under which they were enacted. The exemption appeared to represent early success for biopharma leaders following weeks of engagement with Trump and his administration aimed at staving off tariffs and promoting other industry-friendly policies:
• Pfizer Chairman and CEO Albert Bourla, DVM, PhD—who is also chair of industry group Pharmaceutical Research and Manufacturers of America (PhRMA)—said last month his company could carry out additional drug production in the United States, where it operates 13 manufacturing sites, in order to get around tariffs.
• Bourla and PhRMA president and CEO Stephen J. Ubl led a February 20 meeting of industry leaders with Trump
• David A. Ricks, Lilly chair and CEO, and Amgen chairman and CEO Robert A. Bradway in recent weeks have both publicly credited Trump’s 2017 tax cuts with enticing them to build their manufacturing sites in the United States
“He delivered the tax reform and we delivered the investment in creating high-paying attractive science-based jobs as a result,” Bradway said at the groundbreaking for Amgen’s Holly Springs manufacturing expansion.
Biopharma funds slide with Wall Street
The tariff announcements sparked headline-grabbing stock selloffs in financial markets worldwide that led to low- to mid-single-digit declines Thursday of 2.8% (Japan’s benchmark Nikkei 225 index), 4% (Dow Jones Industrial Average), 4.8% (S&P 500), and 5.97% (Nasdaq Composite). The U.S. markets finished with their worst one-day declines since March 16, 2020, days into the COVID-19 pandemic. The Wall Street slide continued into Friday with declines of 2.75%, 4.96%, 5.5%, and 5.48%, respectively.
However, five of the top six biotech electronic transfer funds (ETFs) as ranked by VettaFi finished last week with double-digit losses between March 28 and Friday. The largest ETF, iShares Biotechnology ETF (NASDAQ: IBB) fell 10.1% from $130.29 to $117.16, compared with a 12.8% loss for the second largest ETF, SPDR S&P Biotech ETF (NYSE Arca: XBI), from $84.40 to $73.63.
The third-largest biotech ETF, First Trust NYSE Arca Biotechnology Index Fund (NYSE Arca: FBT) slid 10.2%, from $170.12 to $152.75, while the fourth-largest, ARK Genomic Revolution ETF (CBOE: ARKG) slipped 11.8% from $21.55 to $19.
Two notable exceptions arose at both ends of the price spectrum. Falling worse than the top four ETFs was the number five ETF, Direxion Daily S&P Biotech Bull 3X Shares (NYSE Arca: LABU), which nosedived nearly 36%, from $69.07 to $44.45. Faring best among the top six ETFs was VanEck Pharmaceutical ETF (NASDAQ: PPH), the sixth largest biopharma ETF, which consists of the shares of 25 pharma giants. PPH shares dropped about 7.5% from $90.65 to $83.89.
“We await potential future pharma sector tariffs in the next month or so and subsequent developments,” Risinger added.
He said those future tariffs will show significant impacts on biopharma manufacturers including 21 companies spotlighted by Leerink in a March 30 report.
Biopharma stock declines could intensify, some analysts warned, if Trump follows through on his earlier talk about levying biopharma tariffs, and especially if his administration imposes import duties—an additional possibility that emerged from news reports that the U.S. Department of Commerce may launch an investigation under Section 232 of the Trade Expansion Act of 1962 into whether drug imports by multinational biopharmas threaten national security.
“President Trump could announce pharma sector tariffs, possibly in the next month or so. When potential sectoral tariffs are announced, we’ll have to see if there are specific company exemptions (potentially those committing to significant investments in the United States),” David Risinger, a senior managing director and senior research analyst covering diversified biopharmaceuticals with Leerink Partners, cautioned in a research note.
Reciprocal risks as China, EU respond
“Additionally, if pharma sector tariffs are announced, we see risks from reciprocal actions by ex-U.S. countries.”
China responded Friday, as its Customs Tariff Commission of the State Council imposed an additional 34% tariff on all U.S.-origin goods effective April 10, and the country’s Ministry of Commerce filed a lawsuit with the World Trade Organization (WTO) challenging the reciprocal tariffs.
“China urges the United States to immediately remove unilateral tariffs and resolve differences with trade partners through dialogue,” a Ministry of Commerce spokesperson said in remarks reported by Global Times, an English-language newspaper published by People’s Daily, the official newspaper of the Chinese Communist Party. “China firmly opposes this and will resolutely take countermeasures to safeguard its own rights and interests.”
“There is no winner in a trade war, and protectionism leads nowhere,” the spokesperson added.
Ursula von der Leyen, president of the European Commission, which oversees the European Union, said the EU will take countermeasures against U.S. tariffs in coming days should talks with Trump administration officials fail. The EU is drafting a set of tariffs on up to €26 billion ($28.4 billion) of U.S. goods set to take effect later this month, after Washington enacted tariffs on steel and aluminum on March 12.
“President Trump’s announcement of universal tariffs on the whole world, including the European Union, is a major blow to the world economy,” von der Leyen stated. “The global economy will massively suffer. Uncertainty will spiral and trigger the rise of further protectionism.”
EU member Ireland, where numerous U.S. biopharmas have manufacturing operations, says it continues to engage with the Trump administration under the working assumption that Washington will levy pharma-specific tariffs in the future.
Pharmaceutical exports account for 79% or about €58 billion (nearly $64 billion) of the €72.6 billion ($79.6 billion) in products that Ireland exported to the United States last year. IDA Ireland, the republic’s foreign investment attraction agency, said the Emerald Isle is home to more than 90 pharmaceutical companies that employ 45,000 people. Of those, around 30,0000 work for U.S.-based companies, according to the industry group Irish Pharmaceutical Healthcare Association (IPHA).
“We’re making the point that actually about 80% of what we produce in companies here that goes into the United States, from a pharma point of view, aren’t finished products,” Ireland’s Deputy Prime Minister or Tánaiste Simon Harris, the country’s Minister for Foreign Affairs and Trade, told Irish broadcaster RTE. “They’re commodities that actually go back into U.S. factories, create jobs for people to pay taxes there.”
Leaders and laggards
- Aldreya Therapeutics (ALDX) shares cratered 73% from $5.33 to $1.42 Thursday after the company announced that the FDA sent the company a complete response letter in response to its second submission of the New Drug Application (NDA) of reproxalap, a candidate designed to treat dry eye disease. The FDA contended that the NDA “failed to demonstrate efficacy in adequate and well-controlled studies in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well-controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted. The letter also cited methodological issues with trial data, including a difference in baseline scores across treatment arms. “Pending positive results from the ongoing clinical trials and discussions with the FDA, we look forward to a potential NDA resubmission mid-year 2025,” stated Todd C. Brady, MD, PhD, Aldreya’s president and CEO. The FDA rejected Aldreya’s first NDA for reproxalap in 2023.
- Allakos (ALLK) shares surged 45% from 22 cents to 32 cents Wednesday, after the company said it had entered into a definitive merger agreement with Concentra Biosciences, which agreed to acquire Allakos for 33 cents a share cash. Allakos’ board has approved the deal, which is subject to the tender of Allakos common stock representing at least a majority of the total number of outstanding shares, the availability of at least $35.5 million of cash (net of transaction costs, wind-down costs, and other liabilities) at closing, and other customary closing conditions. Allakos had been considering strategic alternatives since January.