Weight-loss, diabetes drugs linked to vision problems in small study
Popular drugs for diabetes and weight loss could have an unexpected side effect.
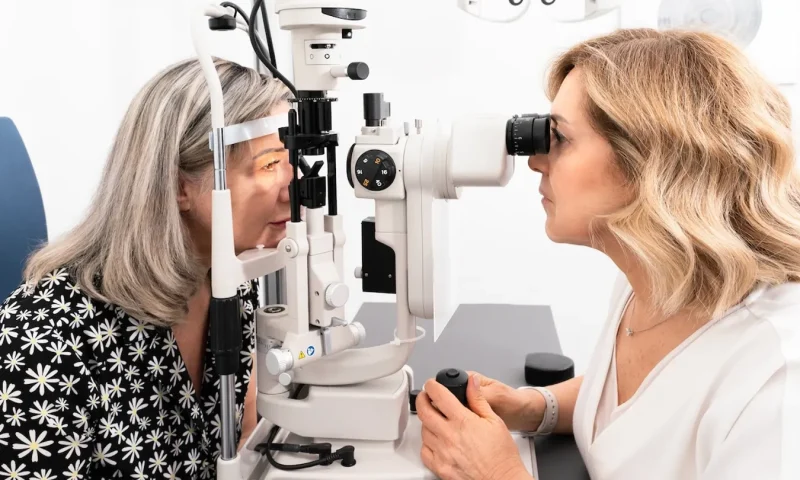
Glucagon-like peptide 1 (GLP-1) receptor agonists, which are used to treat type 2 diabetes and/or obesity, were linked to vision problems in a small study at the University of British Columbia.
Some common GLP-1 drugs include Ozempic and Wegovy, which contain semaglutide as the active ingredient, and Mounjaro and Zepbound, which contain tirzepatide.
In the study, nine patients who were using a GLP-1 developed “ophthalmic complications,” according to the researchers. The average age of the patients was 57.4 years, according to the study findings.
Seven of the patients had nonarteritic anterior ischemic optic neuropathy (NAION), which causes vision loss in one eye.
One patient developed bilateral papillitis, which involves swollen optic nerves that can cause impaired vision, and another had paracentral acute middle maculopathy, which leads to a blind spot in the retina.
All the patients had a history of type 2 diabetes, hyperlipidemia (high lipids or fats in the blood), hypertension and/or sleep apnea.
The findings were published in JAMA Ophthalmology.
“In one of the cases presented, the patient was taking the drugs for weight loss and did not have a prior history of diabetes (which can also be linked to the condition),” Mahyar Etminan, associate professor of medicine at the University of British Columbia, told Fox News Digital. (Etminan was author of the commentary on the study.)
“In another case, when the drug was stopped and reintroduced, the condition reappeared, strengthening a causal link.”
Ziyad Al-Aly, a clinical epidemiologist at Washington University in St. Louis, was not involved in the study but shared his comments on the findings.
“This is a very small study and it was uncontrolled — meaning it did not include people who were not using GLP-1 drugs,” he told Fox News Digital.
“The story of GLP-1 is still being written — and we are learning something new about these drugs every day.”
“This makes it impossible to know whether the reported eye problems are caused by these drugs.”
Nevertheless, the doctor noted, “the story of GLP-1 is still being written — and we are learning something new about these drugs every day. The findings in this study should be pursued further.”
Etminan also acknowledged the study’s limitations.
“This data was derived from a series of individual cases and was not an epidemiologic study,” he noted. “However, another recent epidemiologic study also confirmed an increase in risk.”
Al-Aly called for large, controlled studies — including people who take the drug and a control group of people who are not using the drug — to evaluate the long-term health effects of these medications, including eye problems.
“In the meantime, for people who may be at risk of vision problems, or who already have vision problems, caution is advised,” he added. “People should discuss with their doctors to determine if GLP-1 is the right medication for them.”
Etminan echoed that cautionary guidance.
“Those taking these drugs for diabetes should probably continue taking them for their cardiovascular benefits, but be aware of the signs of NAION,” he advised.
“Healthy individuals taking them to lose a few pounds for an event might want to more carefully weigh the risks versus the benefits of taking these drugs.”
“Most of the vision side effects appear to resolve when the medication is stopped.”
Dr. Seth Kipnis, medical director of bariatric and robotic surgery at Hackensack Meridian Jersey Shore University Medical Center, noted that there have been “rare and unusual side effects” from this class of medications, but he believes the vision changes seem to be more related to the rapid blood sugar changes caused by the medications than to the medications themselves.
“We have encouraged any patients who are on these types of medications to report any unusual symptoms to their prescribing doctors,” Kipnis, who also was not involved in the research, told Fox News Digital.
“Most of the vision side effects appear to resolve when the medication is stopped.”
Kipnis emphasized that these drugs should only be taken under the care of a healthcare professional and that “good and consistent follow-up for dose adjustments with monitoring for side effects” is critical.
When contacted by Fox News Digital, Novo Nordisk (maker of Ozempic and Wegovy) provided the following statement.
“NAION is a very rare eye disease, and it is not an adverse drug reaction for the marketed formulations of semaglutide (Ozempic®, Rybelsus®and Wegovy®) as per the approved labels. After a thorough evaluation of studies from the University of Southern Denmark and Novo Nordisk’s internal safety assessment, Novo Nordisk is of the opinion that the benefit-risk profile of semaglutide remains unchanged.”
The company also noted that eye conditions are “well-known comorbidities” for people living with diabetes.
“Any decision to start treatment with prescription-only medicines should be made in consultation with a healthcare professional who should do a benefit-risk evaluation for the patient in question, weighing up the benefits of treatment with the potential risks,” Novo Nordisk added.