In an interview at the J.P. Morgan Healthcare Conference earlier this year, Viz.ai CEO Chris Mansi, M.D., told Fierce Medtech that the company is aiming to increase its current suite of disease-spotting artificial intelligence tools—a handful of which have already been cleared by the FDA—more than tenfold in the next three to five years.
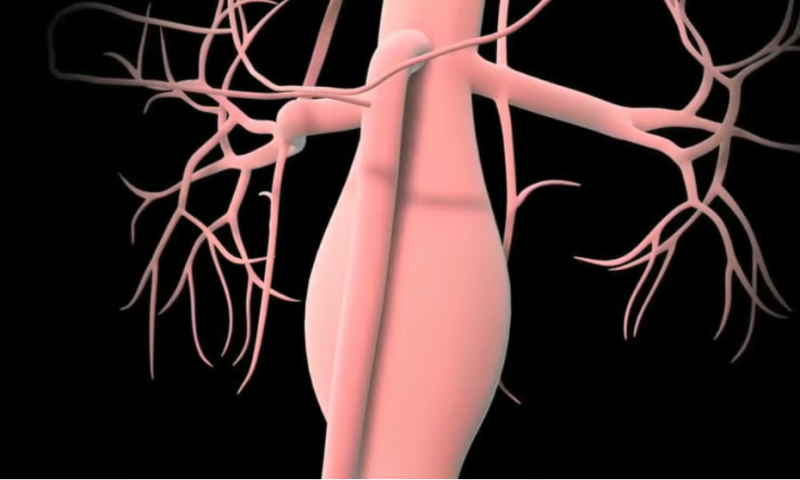
In its latest step toward that goal, Viz has secured the FDA’s clearance for yet another of its AI algorithms. This one, according to a Tuesday announcement, is designed to look for signs of an abdominal aortic aneurysm (AAA), in which the section of the aorta that runs through the stomach begins to bulge or swell.
The condition is estimated to affect about 5% of people around the world and to cause more than 175,000 deaths globally each year, most commonly when the aneurysm goes undetected, allowing the blood vessel to swell too far and rupture.
“AAA is an important and actionable incidental finding that is too often missed,” Jayme Strauss, Viz’s chief clinical officer, said in the announcement. “The vision behind Viz AAA is to automatically catch and follow abdominal aortic disease, no matter the patient’s location. We believe that this product will enable care teams to prevent catastrophic aortic emergencies, such as aortic rupture, by increasing the surveillance of these patients.”
The FDA clearance makes Viz’s AI tool the first to be given the agency’s green light to detect and triage suspected cases of AAA, according to the company, which was named to Fierce Medtech’s annual Fierce 15 list this week.
Viz AAA has been trained to look for signs of aneurysm in CT angiography scans of the chest—spanning any CTA scan, no matter the scanner used to capture it, according to Viz—and automatically flag the patient’s care team if a possible aneurysm is detected.
The algorithm will fold into the FDA-cleared Viz Aortic module, which is housed on the company’s AI-powered platform and combines several of its aortic disease-detecting algorithms in one place. To date, the module includes AI to spot both Type A and Type B aortic dissections, along with the new AAA-focused technology, plus additional tools not powered by AI to help doctors identify thoracic abdominal aneurysms and ruptured aneurysms.
Late last year, Viz presented study results highlighting its deep learning AI’s ability to spot aortic dissections. According to the study, when the algorithm was tasked with analyzing more than 1,300 CTA scans, it correctly detected aortic dissections with about 94% sensitivity, while excluding negative cases with 97% specificity.
The latest FDA nod comes shortly after Viz announced yet another new addition to its portfolio. In that case, Viz has reeled in support from Bristol Myers Squibb for an AI algorithm that looks for signs of hypertrophic cardiomyopathy.
Viz has already built the algorithm—dubbed Viz HCM—and submitted it to the FDA for review, according to the announcement earlier this month, and didn’t disclose the specifics of BMS’ involvement in the project.