As colleagues at Seattle Children’s Hospital, Andy Scharenberg, M.D., and Michael Jensen, M.D., knew they wanted to build a startup with a mission.
Although cell therapies like Novartis’ Kymriah and Gilead’s Yescarta have transformed treatment for certain blood cancers, Scharenberg, a pediatric immunologist by training, was struck by the numbers of patients who can’t get those treatments.
“I wanted to create something where the impact would be absolutely the biggest possible swing you could take,” he said.
That was the driving force behind the pair’s decision to found Seattle-based Umoja Biopharma, which debuted Tuesday with a $53 million series A round. Scharenberg envisions Umoja, which means “unity” in Swahili, as a unification of three approaches developed by scientists at Seattle Children’s and Purdue University.
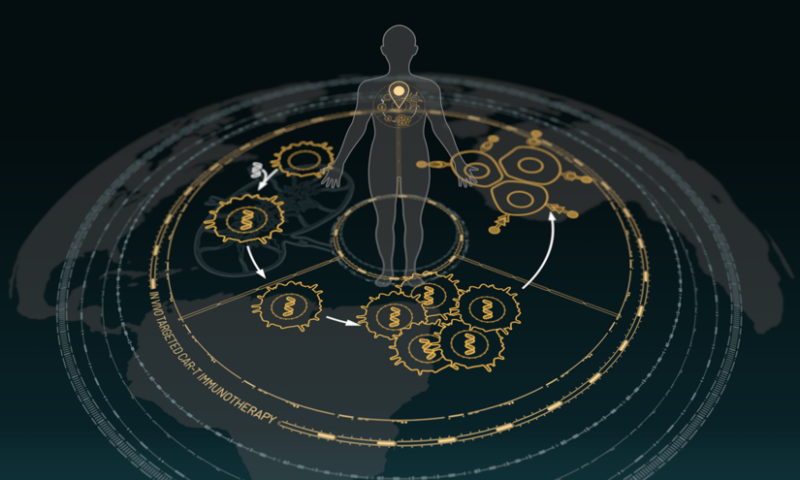
Umoja’s multipronged approach relies on three platforms used in sequence.
“We’re going to give you a first medicine that’s going to grow a cancer-fighting cell army in your body, and then we’re going to send that cancer-fighting army in your body messages telling it how to kill off your tumor,” Scharenberg said.
That first medicine is VivoVec, which generates cancer-fighting CAR-T-cells inside the body, boosting the immune system. This approach is different to that of current treatments Kymriah and Yescarta, which require T cells to be extracted from patients, engineered outside the body and then given back to the patient to fight cancer. VivoVec doesn’t need “pre-conditioning”—a treatment used to clear a path for cell therapies to work but that tends to suppress the immune system—and it mimics the body’s own immune response, reducing the risk of cytokine release syndrome, a common side effect of CAR-T treatments.
After two weeks to allow for the number of T-cells to expand in the body, the patient would then receive TumorTag, which binds to both tumor cells and the “shields” they use to hide from the body’s natural immune defenses. As its name suggests, the technology tags them as targets for the newly created army of T-cells.
Finally, the patient would receive RACR/CAR, which doctors can use to tune the activity of the engineered T cells in the body with the help of FDA-approved drugs. One of those drugs is rapamycin, an immunosuppressant.

Umoja analyzed 15 companies in a similar space, finding that “each individual company was doing one of our platforms,” Scharenberg said. It decided to combine multiple technologies, designing them from the ground up to work together.
Scharenberg sees immunotherapies as the future of cancer therapies because, in pediatrics, chemotherapy and radiation can cause “devastating” lifelong developmental issues. In adults, immunotherapies avoid “chemo fog” and other side effects associated with chemotherapy and radiation.
Umoja plans to use its funds, led by MPM Capital and Qiming Venture Partners USA, to continue building out its team and focus on two indications: blood cancer and pediatric osteogenic sarcoma, a type of bone cancer in children. Both have limited treatment options.
Scharenberg hopes to start clinical trials for the pediatric indication in the second half of next year at Seattle Children’s.