More than a year after doling out an emergency use authorization for a handful of Philips’ IntelliVue patient monitoring devices, the FDA is doubling down on the regulatory nod.
Two of the patient monitors included in the June 2020 EUA have now received 510(k) clearance, permitting them to be marketed even after the agency has determined the ongoing public health emergency caused by the COVID-19 pandemic to be over.
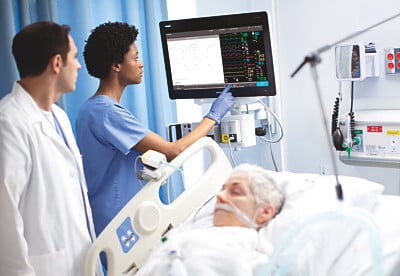
Both the MX750 and MX850 models of the IntelliVue devices offer continuous patient monitoring in the intensive care unit and beyond. Healthcare providers can set custom alerts based on changes in each patient’s vital signs, integrate the patient’s electronic health record into the monitor’s display and connect the monitoring system to ventilators, anesthesia machines, IV pumps and other devices.
The two bedside monitors—which Philips said are the most advanced yet of the IntelliVue collection—are also designed with hospitals’ and patients’ cybersecurity concerns in mind. They feature encryption services for the remote display data and all other information gathered by the devices, including file system, print report and remote control command encryption.
Both of those models, plus a pair of IntelliVue add-on display screens and a module server rack to organize the IntelliVue system, were included in the emergency authorization last summer. At the time, the FDA determined that the remote monitoring technology was immediately and critically needed amid the surging coronavirus pandemic.
In its announcement about the EUA, Philips said it was planning on submitting the devices for full FDA clearance before the end of 2020.
Meanwhile, the monitors and displays have been cleared in Europe since 2019, when they received a CE mark and promptly began rolling out to hospitals on the continent. The new 510(k) clearance includes just the MX750 and MX850 devices, while the active displays and server rack are still covered in the U.S. only by the emergency nod.
“Continuous patient monitoring plays a vital role in overall patient safety while providing clinicians and caregivers with the holistic view they need to best support their patients and manage their workload,” said Sandra Lesenfants, general manager of Philips’ hospital patient monitoring business.
The two monitors, Lesenfents said, are “a key part of our modular portfolio of hardware, software, services and consumables that can be tailored to provide integrated monitoring solutions that meet the individual needs of each healthcare provider.”
The FDA clearance is a bright spot amid a rocky year for the Dutch medtech giant. Though the initial emergency clearance of the patient monitoring system brought a boost to last year’s sales numbers in Philips’ connected care division, the segment has struggled this year due to a far-reaching Class I recall of many of its CPAP machines and other ventilators.
In the third quarter of this year—the first to feel the full effects of the recall after it was initiated in June—connected care sales plummeted nearly 40% from 2020’s numbers. That drop sent shockwaves across the entire company, which reported an overall year-over-year decrease of more than 7% in global sales for the quarter.