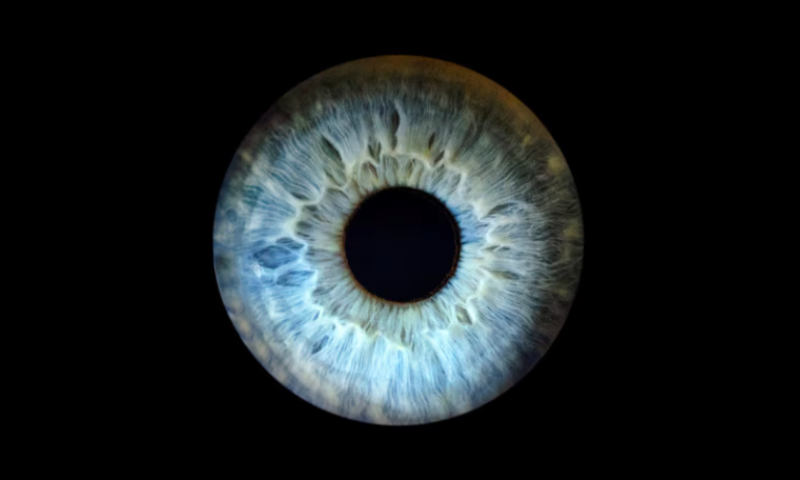
Nuvation’s slimmed down solid tumor strategy is already facing difficulties, with the FDA slapping a partial clinical hold on the biotech’s drug NUV-422 following reports of eye inflammation.
The CDK2/4/6 inhibitor is currently undergoing a phase 1 study to explore a maximum tolerated dose. An unspecified number of patients have reported uveitis—a form of inflammation of the middle layer of the eye—leading the biotech to pause enrollment of new patients and reach out to the regulator for guidance.
The FDA decided that while no new patients will be enrolled in the NUV-422 program, current participants may continue to be treated, Nuvation said.
“Based upon the recent development of uveitis as a potential safety signal, we will conduct an overall risk/benefit analysis of the NUV-422 program,” Nuvation Bio CEO David Hung, M.D., said in a June 27 announcement. “With $737.7 million in cash as of March 31, 2022, we are well positioned to continue developing all of our internal product candidates.”
The company will provide updates on the direction of the NUV-422 program after completing the internal risk-benefit analysis, which will factor in feedback from the FDA.
The early-stage trial involves testing NUV-422 against a range of solid tumors, including high grade glioma, HR+/HER2- advanced breast cancer and metastatic castration-resistant prostate cancer. A total of 269 participants had been recruited as of March 11, according to ClinicalTrials.gov.
Nuvation narrowed its solid tumor portfolio in May, de-prioritizing three less-advanced assets and moving NUV-422 and its BET inhibitor NUV-868 onto center stage.
The company’s shares declined about 8% to $3.83 as the markets opened Monday morning, compared to a prior close of $4.19.