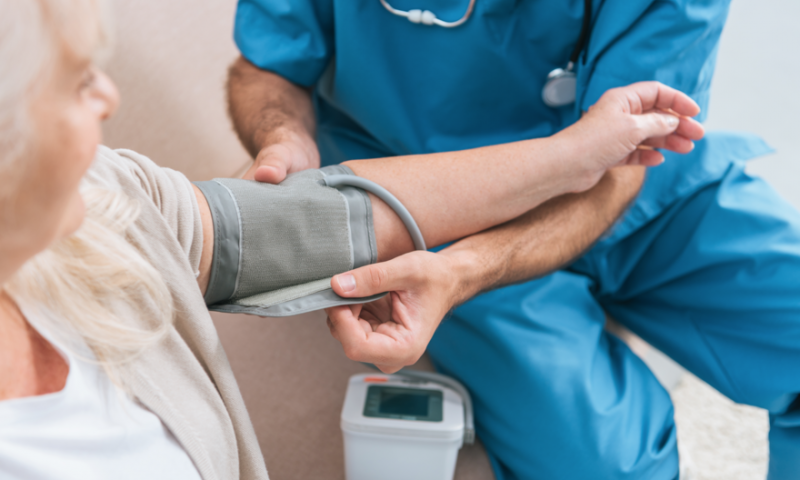
Medtronic’s years-long effort to develop renal denervation into a therapy continues, with promising new data from a three-year registry. More than 2,500 international patients with uncontrolled high blood pressure showed real-world improvements in their numbers following treatment with the medtech giant’s Symplicity system, regardless of any heart medications they were taking.
The procedure demonstrated significant drops in office-measured systolic blood pressure, by 17 mmHg, leading to more patients achieving a blood pressure of below 140. Meanwhile, those with very high levels of hypertension saw their readings fall into ranges associated with fewer health risks.
Additionally, the total number of patients in the registry with the highest blood pressures—more than 180 mmHg at the start—decreased by two-thirds over the three-year period. All this occurred while the participating patients were prescribed an average of four or more medications.
The data, delivered during the virtual PCR e-Course 2020 program, follow positive Symplicity results presented earlier this year at the annual meeting of the American College of Cardiology, held together with the World Congress of Cardiology.
There, a sham-controlled study of Medtronic’s updated Spyral model—featuring four spaced-out electrodes for more even ablation of the nerves near the kidneys—showed average drops of 6.5 mmHg after three months. Participants also saw average reductions of 3.9 mmHg at home over a 24-hour period, measured by a wearable blood pressure monitor.
“As the body of clinical evidence supporting renal denervation grows, we are encouraged by the outcomes observed in both controlled clinical trials and real-world practice,” said Dave Moeller, general manager of Medtronic’s coronary and renal denervation business.
The Symplicity Spyral system has not yet been approved for use in the U.S., and is being studied both with and without hypertension medications.