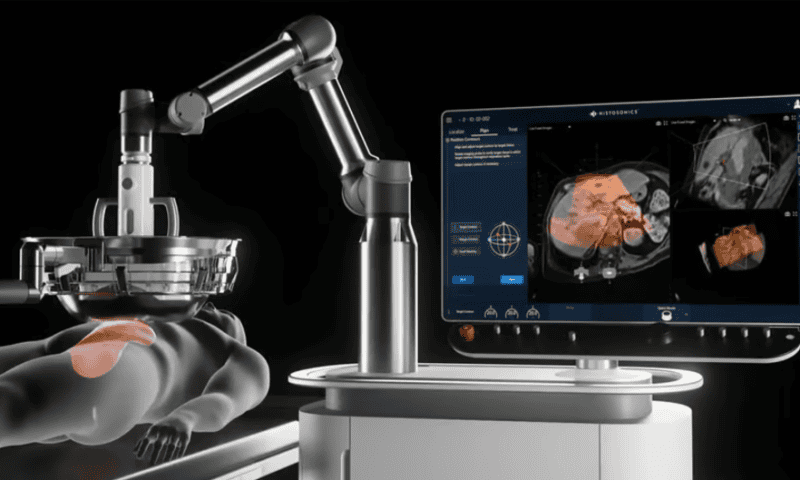
After collecting a de novo clearance from the FDA last fall for its ultrasound-powered liver cancer treatment, HistoSonics has raised $102 million in funds to turn up the volume on its commercial efforts.
The company’s Edison system uses the vibrations from focused pulses of sound waves to create bubbles of gas within a tumor—beaming enough energy to ultimately liquify and destroy the target at the cellular level while sparing the surrounding tissue.
The former Fierce 15 winner’s series D round was led by Alpha Wave Ventures and was joined by Amzak Health, HealthQuest Capital, Johnson & Johnson Innovation, Venture Investors, Lumira Ventures, Yonjin Venture, and the State of Wisconsin’s investment board among others.
“Histotripsy is a paradigm-changing treatment option for patients who want a non-invasive approach to target and destroy tumors without the need for needles or incisions,” HistoSonics President and CEO Mike Blue said in a statement.
“We’re thrilled to announce this top-tier investor syndicate led by Alpha Wave, which reinforces the confidence in our mission to impact patients’ lives with our current liver application and expanded use in kidney, pancreas, prostate, brain and other tumor types,” Blue added.
“This funding will accelerate key projects designed to enhance core technical capabilities impacting current and future platforms, and support collaboration with physicians and researchers studying innovative ways to use histotripsy’s unique mechanism of action to improve patient outcomes.”
The proceeds will also help launch the company’s BOOMBOX master study, which will evaluate Edison’s use against liver cancer among multidisciplinary healthcare providers. It also plans to double the size of its Minneapolis, Minnesota-area headquarters, according to a report from the Star Tribune.
Earlier this year, HistoSonics reported that its first patients with liver cancer were treated at the University of Rochester Medical Center in New York and at the Cleveland Clinic, following its FDA green light.
Shortly thereafter, the company said it treated its first patient with kidney cancer through an ongoing clinical trial, at AdventHealth Celebration in Florida.