Oregon Medical Research Center in Portland, Oregon, has led a phase 2 trial in moderate-to-severe plaque psoriasis that paired higher-than-approved doses of risankizumab with extended follow-up and found high early skin clearance alongside reduced lesional tissue-resident memory T cells at Week 52.
Psoriasis relapse
Psoriasis is a common, chronic, immune-mediated inflammatory disease. Many highly effective medications exist, but the disease eventually returns after stopping treatment when tissue resident memory T cells, persisting in previously affected skin, restart inflammatory activity.
Risankizumab targets interleukin-23 and is described as highly effective and safe for treating moderate-to-severe plaque psoriasis. Approximately 80% of patients achieve greater than 90% clearance of skin lesions at one year, and approximately 60% reach complete skin clearance at one year, with few-to-no side effects.
Previous clinical results found prolonged skin clearance after stopping risankizumab in some patients. Approximately 10% of patients with completely clear skin after three risankizumab 150 mg doses maintained completely clear skin for up to one year before psoriasis recurred. Phase 1 trial results also showed long-term clearance of lesions after a single high dose. Risankizumab is postulated to induce high levels of prolonged skin clearance due to an effect on tissue resident memory T cells (TRM).
The two faces of TRM
TRM develop within tissues in response to pathogen exposure, are long-lived, and protect skin from re-exposure to the original pathogen. TRM are also found in skin previously affected by active psoriasis and are believed to be responsible for recurrences.
Recent evidence suggests that psoriatic tissue TRM are under the control of interleukin-23 and risankizumab has been studied as an anti-interleukin-23 biologic.
Trial plan and clinical follow-up
In their study, “A randomized phase 2 clinical trial to treat moderate-to-severe plaque psoriasis patients with high-induction dosing of risankizumab,” published in Nature Communications, the researchers conducted the KNOCKOUT study as a single-center, randomized, double-blinded, 100-week, interventional Phase 2 trial to measure change from baseline TRM. Evaluation also looked into whether higher doses could produce high levels of complete skin clearance and long-term remissions.
A total of 20 patients were randomized, with 10 receiving 300 mg and 10 receiving 600 mg of subcutaneous risankizumab at Weeks 0, 4, and 16. Monitoring was attempted through Week 100 without additional dosing. Of the participants, 16 made it to Week 52 and only six made it to Week 100.
Biopsies down to single cells
Skin biopsies from lesional and non-lesional sites were collected at baseline, with lesional biopsies collected again at Week 52 in 13 of the remaining 16 participants. Tissue was processed into single-cell suspensions for scRNA-seq, splitting each 6 mm biopsy and cryopreserving a portion before enzymatic digestion.
Single-cell profiles supported classification of T cell subtypes and comparison of tissue resident memory T cell populations before and after treatment. Efficacy reporting used modified non-responder imputation, excluding patients who did not receive all three induction doses for reasons other than lack of efficacy or intolerability.
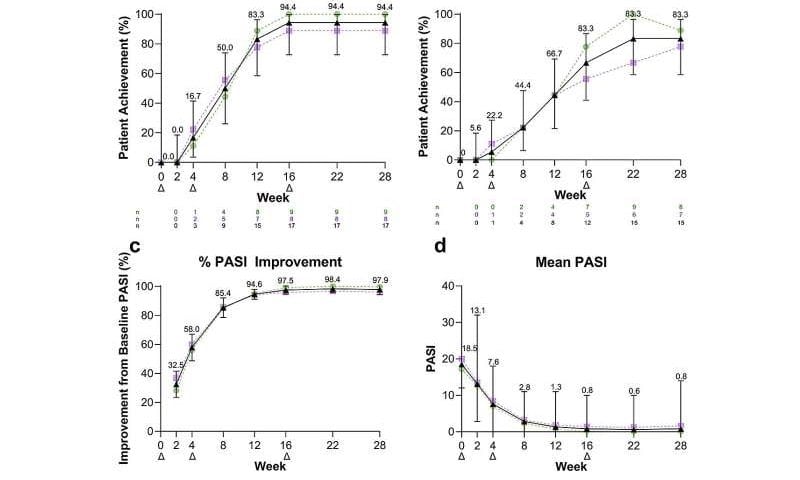
Week 52 skin clearance used the Psoriasis Area and Severity Index, or PASI. PASI 75 and PASI 90 mark escalating clearance thresholds, with PASI 100 signifying completely clear skin.
Week 16 PASI 75 was 100%, PASI 90 was 94.4%, and PASI 100 was 66.7%. Week 28 PASI 75 and PASI 90 were 94.4%, and PASI 100 was 83.3%. DLQI 0 or 1 occurred in 88.9% at Week 28. Week 52 was measured 36 weeks after the last risankizumab dose. PASI 75 was 77.8%, PASI 90 was 61.1%, and PASI 100 was 44.4%.
Week 52 skin samples showed fewer tissue resident memory T cells that had been living inside psoriasis plaques. One subgroup, CD8 TRM17, dropped from an average of 22 cells per sample at lesional baseline to an average of 3 cells per sample in the 600 mg group at Week 52.
IL-17 signaling gene activity went down in CD8 TRM17 cells after risankizumab treatment, including IL17A, IL17F, and IL22. Computational predictions suggested weaker signaling between keratinocytes and CD8 TRM cells at Week 52, including predicted reductions in IL-15 to IL-15R, IL-7 to IL-7R, and TNFSF15 to TNFRSF25. Larger predicted reductions appeared in the 600 mg group than the 300 mg group.
Researchers concluded that high induction dosing of risankizumab produced rapid, durable skin clearance in moderate-to-severe plaque psoriasis with no new safety signals observed, alongside a marked reduction of TRM within the limited number of long-term participants.