A study of Fractyl Health’s one-time procedure for rejuvenating the lining of the intestines demonstrated significant and durable gains in blood sugar control for up to two years among people with Type 2 diabetes.
The open-label trial followed 34 people in Europe and South America and found no serious effects. At the same time, the company said participants saw reductions in weight and logged improvements among self-reported measures of diabetes therapy satisfaction.
The study included patients with poorly controlled diabetes despite taking at least one glucose-lowering medication but who had not yet begun insulin therapies.
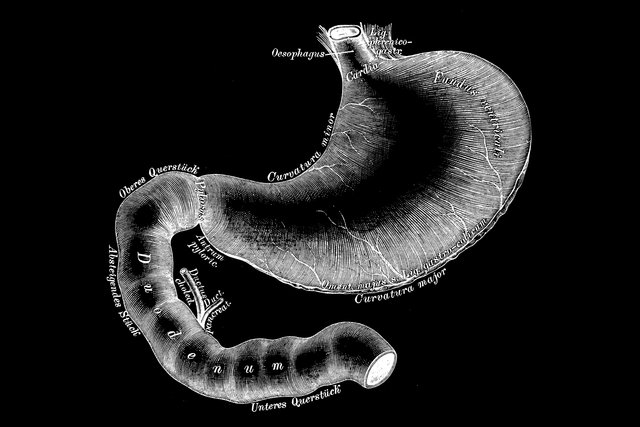
Fractyl’s endoscopic Revita DMR device strips out and helps reset the inner mucosal lining of the duodenum by applying heat through a water-filled balloon catheter. This intestinal layer can become thickened by years of dietary fats, sugars and other foods, and Fractyl believes this contributes to the insulin resistance and metabolic imbalances linked with Type 2 diabetes.
Two years after a Revita procedure, the study saw an average one percentage point reduction in HbA1c levels, down to 7.5% from a baseline reading of 8.5%. In addition, most patients either reduced or maintained the number of their diabetes prescriptions, according to the company.
Meanwhile, average weight loss was measured as 3.1 kilograms, and patients also saw an increase in their HDL—or “good” cholesterol—levels by an average of 6.4 points. Changes in triglycerides, LDL levels and total cholesterol were not statistically significant. The results were published in the journal Diabetes Research and Clinical Practice.
Last year, Fractyl secured $100 million in venture capital to help carry its device across the finish line at the FDA. It had previously garnered a breakthrough tag and a CE mark in Europe. In 2020, the company raised $55 million to help power its clinical trials.