The FDA is upgrading its safety warnings related to complications from Hologic’s implantable marker for breast cancer procedures, after making an alert to the public earlier this year.
This week, the agency handed down a Class I recall label to the issue, its most serious designation, following 71 reports of patient injuries. The notice does not require any hardware to be returned to the manufacturer and it may continue to be used, including some 53,000 devices distributed across the U.S., according to the company and the FDA.
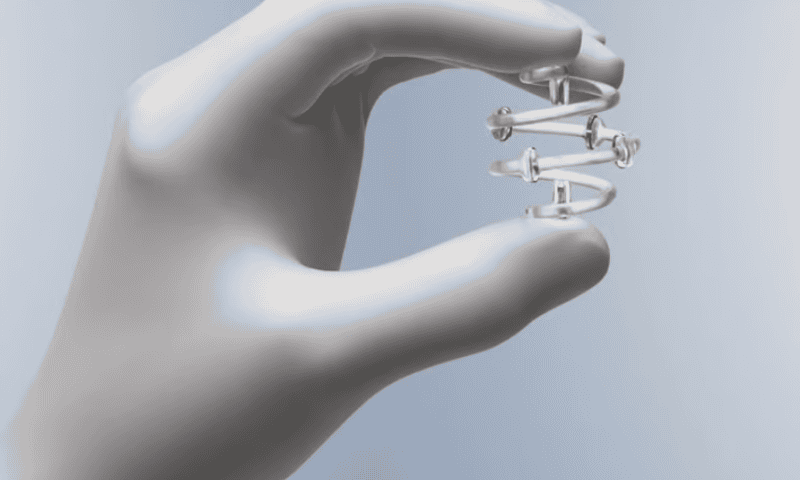
The company’s dissolving BioZorb implants are used to mark locations in soft tissue for future reference—for example, to designate the site of a lumpectomy for follow-up imaging exams or additional radiation. It includes a three-dimensional coil made out of an absorbable material, as well as six permanent titanium clips.
In late February, the FDA issued a safety communication that referred to an undisclosed total number of patient reports.
The agency said that complaints have included reports of pain, infections and rashes, as well as cases where the device has moved or broken through the skin, or caused build-ups of fluid within the breast.
The FDA said some patients required additional procedures to remove the implant, and that certain complications are “possibly associated with extended resorption time,” with the BioZorb’s coil taking several years to dissolve.
Hologic sent instructions to customers in mid-March, telling patients to contact their provider if they experience any side effects, and that clinicians should discuss the risks of implantable markers prior to any breast cancer procedures or breast-conserving surgeries.
The company acquired the BioZorb implant line through its $125 million buyout of Focal Therapeutics in late 2018.
Previous Hologic marketing materials (PDF) included descriptions of a surgeon using BioZorb’s spherical shape to “support tissue healing and breast contour.” However, the FDA in its notices underlined that the device has not been approved “to fill space in the tissue or to improve cosmetic outcomes after procedures.”
The FDA said it will continue to work with Hologic to evaluate the implant’s safety and monitor reports of adverse events.