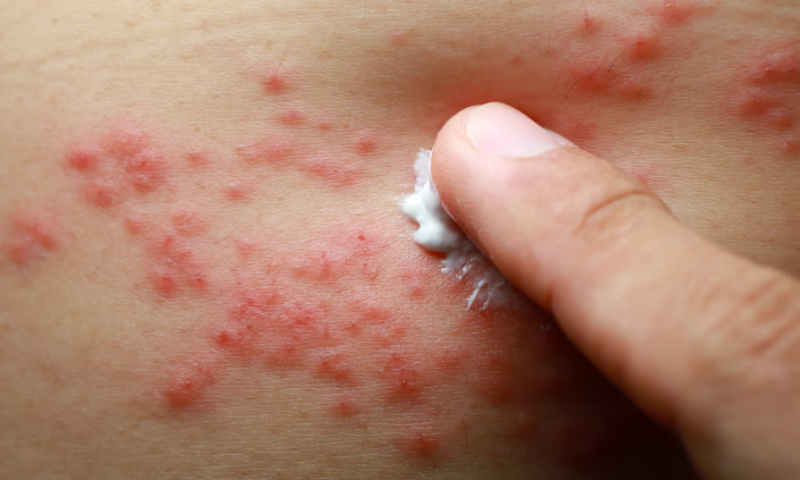
In a sign that the tough climate for biotechs will last into 2023, Connect Biopharma cited the icy funding environment for its decision to halt a global phase 3 program for its lead dermatitis asset while it seeks out a suitable partner.
The IL-4Rα cytokine receptor-targeting antibody, dubbed CBP-201, remains in a phase 3 trial for atopic dermatitis in China as well as a midstage study for asthma. But Connect said last Friday that a planned global phase 3 trial in dermatitis, which was due to launch before the end of 2022, would no longer go ahead until a partner is found.
“We continue to have great confidence in our global development strategy, and particularly in the potential of our lead product candidate CBP-201,” CEO Zheng Wei, Ph.D., said in a Dec. 30 release. “Yet, in light of the current macroeconomic climate and challenging funding environment, we feel that it is necessary and financially prudent to commence our global phase 3 program for CBP-201 in moderate-to-severe AD after we have secured the partnership necessary to fully complete the program.”
When it comes to finding the perfect partner, Connect said in the release that it’s looking to hook up with companies with the “additional experience and infrastructure to support the next phase of clinical development for CBP-201, including providing potential input into the clinical trial designs.”
The strategy for CBP-201 in China remains on track despite the halt elsewhere, the company stressed. Connect is aiming for meetings with Chinese regulators in the next three months to discuss the data required for a potential approval application in 2024. If all goes to plan, the biotech is hoping for a green light in the country in 2025.
Meanwhile, top-line results of CBP-201’s asthma trial are expected to read out in the second half of the year.
The strategic pause for CBP-201’s global dermatitis plans should free up cash to fund the company for at least an additional year, meaning the biotech is now financially secure into 2025. “We anticipate that this longer runway will allow us to meet the milestones for our ongoing clinical trials and advance our preclinical assets toward the clinic, while we continue to evaluate partnership opportunities,” Chief Financial Officer Steven Chan said in the release.
That pipeline also includes CBP-307, a modulator of the sphingosine 1-phosphate receptor. The candidate’s journey hasn’t been without hiccups—the company tried to spin a positive story out of the therapy’s failure to hit a primary endpoint in May last year—while a midstage trial in Crohn’s disease was halted early due to COVID-19-related enrollment challenges. Connect said it’s “actively seeking to out-license CBP-307” for future trials in colitis and Crohn’s.
The biotech’s remaining clinical candidate is CBP-174, an H3 receptor antagonist that completed a phase 1 study in pruritus associated with allergic and inflammatory skin diseases back in August. The company is “continuing to evaluate next steps for clinical development,” it added.