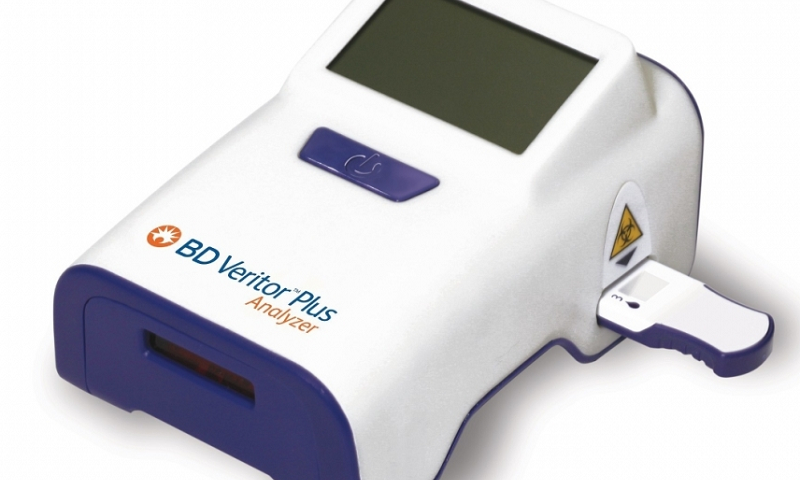
The FDA granted an emergency authorization to a portable coronavirus antigen test developed by BD, similar to a rapid flu test, designed to allow hospitals, doctors’ offices, urgent care centers and retail pharmacies to examine a person showing symptoms in about 15 minutes.
At the same time, the agency alerted healthcare providers of potential false-positive results from a separate BD laboratory diagnostic for COVID-19, which previously received an FDA green light in April.
The new hand-held test runs on the Veritor Plus system, with a device about the size of a cellphone, using nasal swabs. BD says the platform has already been used at more than 25,000 U.S. locations to test for diseases such as influenza, respiratory syncytial virus and strep throat. The company plans to manufacture 10 million coronavirus tests through September and produce 2 million per week thereafter.
This is the second antigen-based test authorized by the FDA for use during the pandemic, following Quidel’s green light in early May. Antigen tests search for the unique proteins coating the outer walls of the coronavirus, as opposed to molecular tests that examine its genetic structure or antibody tests that gauge the human body’s immune response.
“Such tests will also help communities be more informed and better prepared to help prevent new spikes and additional waves of COVID-19 by enabling public health workers to quickly identify infectious individuals and trace their contacts,” said Dave Hickey, president of Integrated Diagnostic Solutions for BD, which also plans to market the test internationally following additional regulatory approvals.
In clinical studies, the diagnostic correctly spotted 84% of samples positive for COVID-19 while delivering zero false positives, according to BD. The FDA, however, recommends that negative immunoassay results still be confirmed by a molecular test.
On the same day as the Veritor system’s authorization, the FDA said the company’s automated BD MAX molecular laboratory diagnostic could carry a risk of false positives and that healthcare personnel should treat positive readings as presumptive.
One study performed by BD found the test’s reagents lead to a false-positive rate of about 3%, according to the agency, which recommended that results be confirmed with another authorized test. The FDA said it is working with BD to resolve the issue.
BD also previously offered a fingerstick antibody blood test for COVID-19, developed by BioMedomics, but later took it off the market as the companies work to develop a new version for submission to the FDA.