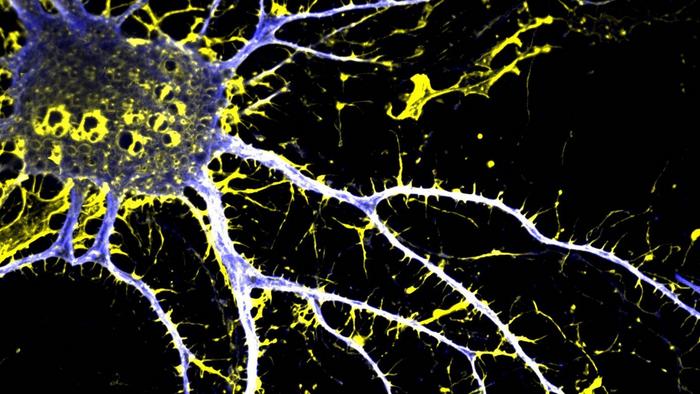
Psychedelic drugs, such as lysergic acid diethylamide (LSD), can increase neuronal growth, which can have therapeutic effects for conditions such as schizophrenia. However, such drugs can induce hallucinations and may worsen some symptoms. Researchers from the University of California (UC), Davis, say they have now developed a new, neuroplasticity-promoting drug, (+)-JRT, that is a close structural analog of LSD and harnesses the psychedelic’s therapeutic power, but which exhibits reduced hallucinogenic potential.
Their research, headed by David E. Olson, PhD, director of the Institute for Psychedelics and Neurotherapeutics and a professor of chemistry, and biochemistry and molecular medicine at UC Davis, highlights the new drug’s potential as a treatment option for conditions such as schizophrenia, for which psychedelics are not prescribed for safety reasons. The investigators’ studies, including tests in rodent models, showed that as well as promoting spinogenesis in the cortex, (+)-JRT produced therapeutic effects in behavioral assays relevant to depression and cognition. The team suggests the new compound may also be useful for treating other neuropsychiatric and neurodegenerative diseases characterized by synaptic loss and brain atrophy.
Olsen and colleagues reported on their development of (+)-JRT in PNAS, in a paper titled, “Molecular design of a therapeutic LSD analog with reduced hallucinogenic potential,” in which they concluded, “Our work highlights the potential of rationally designed, nonhallucinogenic analogs of psychedelics for treating diseases where the use of psychedelics is contraindicated.”
“Decreased dendritic spine density in the cortex is a key pathological feature of neuropsychiatric diseases including depression, addiction, and schizophrenia (SCZ),” the authors wrote. But while psychedelics possess what they describe as a remarkable ability to promote cortical neuron growth and increase spine density, “their hallucinogenic properties have limited their adoption as medicines and preclude their use in certain patient populations, such as those with schizophrenia or a family history of psychosis.”
To design the molecule (+)-JRT the researchers flipped the position of just two atoms in LSD’s molecular structure. The chemical flip reduced JRT’s hallucinogenic potential while maintaining its neurotherapeutic properties, including its ability to spur neuronal growth and repair damaged neuronal connections that are often observed in the brains of those with neuropsychiatric and neurodegenerative diseases.
“Basically, what we did here is a tire rotation,” said corresponding author Olson. “By just transposing two atoms in LSD, we significantly improved JRT’s selectivity profile and reduced its hallucinogenic potential.”
Olson said that it took his team nearly five years to complete the 12-step synthesis process to produce (+)-JRT. The molecule was named after Jeremy R. Tuck, a former graduate student in Olson’s laboratory, who was the first to synthesize it and is a co-first author of the study, along with Lee E. Dunlap, another former graduate student in Olson’s laboratory.
(+)-JRT and LSD have the exact same molecular weight and overall shape, but distinct pharmacological properties. JRT is very potent and highly selective for binding to serotonin receptors, specifically 5-HT2A receptors, the activation of which are key to promoting cortical neuron growth.
Following successful synthesis of (+)-JRT the researchers conducted a battery of cellular and mouse assays that demonstrated the drug’s neuroplastic effects and improved safety profile relative to LSD. Their results showed that (+)-JRT exhibited powerful neuroplastic effects and improved measures in mice relevant to the negative and cognitive symptoms of schizophrenia, without exacerbating behaviors and gene expression associated with psychosis.
In lab experiments, the authors found that (+)-JRT selectively bound serotonin receptors with similar potency to LSD but without activating associated signaling cascades responsible for the hallucinogenic effects. The analog was as effective at stimulating dendrite growth in cultured rat cortical neurons as LSD. Analysis of mouse brain slices revealed that one dose of (+)-JRT led to a 46% increase in dendritic spine density in the prefrontal cortex 24 hours later.
(+)-JRT also did not produce hallucinogenic-like behaviors that are typically seen when mice are dosed with LSD, and did not promote gene expression associated with schizophrenia. Such gene expression is typically amplified with LSD use.
(+)-JRT produced robust antidepressant effects, with it being around 100-fold more potent than ketamine, the state-of-the-art fast-acting antidepressant. In a mouse model of chronic stress, 20 days of corticosterone injections reduced dendritic spine density and induced behavioral changes suggesting the depression of cognitive inflexibility. One dose of (+)-JRT rescued stress-related losses in spine density and behavioral changes. (+)-JRT promoted cognitive flexibility, successfully addressing deficits in reversal learning that are associated with schizophrenia.
“Despite its lower hallucinogenic potential, (+)-JRT has demonstrated profound therapeutic effects,” the team wrote. “… we demonstrate that (+)-JRT possesses unique pharmacological properties as a selective serotonergic agent that can potentially address negative and cognitive symptoms associated with SCZ without exacerbating positive symptoms or producing the side effects characteristic of current treatments.”
Added Olsen, “JRT has extremely high therapeutic potential. Right now, we are testing it in other disease models, improving its synthesis, and creating new analogs of JRT that might be even better.”
Olson emphasized JRT’s potential for treating the negative and cognitive symptoms of schizophrenia, as most current treatments produce limited effects on anhedonia—the inability to feel pleasure—and cognitive function. Clozapine is the one exception, but it has side effects, and is not the first-line drug of choice for people severely afflicted with schizophrenia.
Olson and his team are currently testing the potential utility of (+)-JRT against other neurodegenerative and neuropsychiatric diseases. “No one really wants to give a hallucinogenic molecule like LSD to a patient with schizophrenia,” said Olson, who is co-founder and chief innovation officer of Delix Therapeutics, a company that aims to bring neuroplastogens to the market. “The development of JRT emphasizes that we can use psychedelics like LSD as starting points to make better medicines. We may be able to create medications that can be used in patient populations where psychedelic use is precluded.”
In their paper, the authors concluded, “By transposing only two atoms, we have created JRT, an exceptionally potent analog of LSD with lower hallucinogenic potential, improved pharmacological selectivity, and the ability to produce a wide range of therapeutic effect … Taken together, our work highlights the potential for modifying the chemical structures of psychedelics to produce analogs with improved efficacy and safety profiles and further underscores the potential usefulness of nonhallucinogenic psychoplastogens for treating illnesses unlikely to be addressed by psychedelics such as SCZ, bipolar disorder, and psychosis in neurodegenerative diseases.”