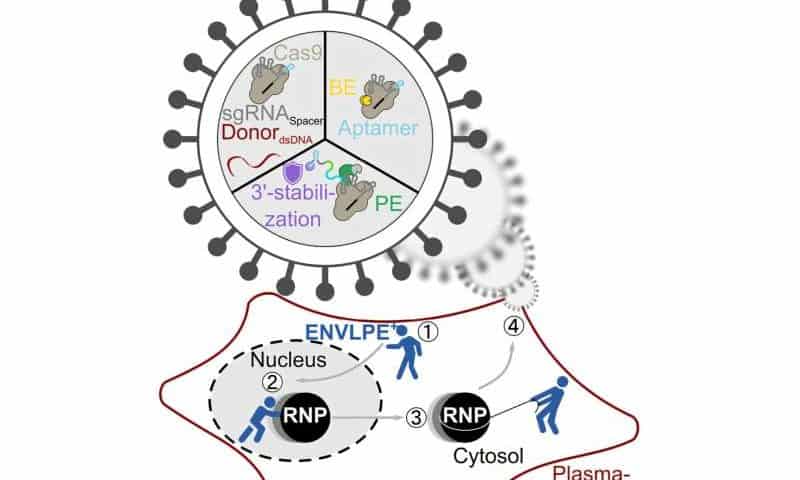
A research team from Helmholtz Munich and the Technical University of Munich has developed an advanced delivery system that transports gene-editing tools based on the CRISPR/Cas9 gene-editing system into living cells with significantly greater efficiency than before. Their technology, ENVLPE, uses engineered non-infectious virus-like particles to precisely correct defective genes—demonstrated successfully in living mouse models that are blind due to a mutation.
This system also holds promise for advancing cancer therapy by enabling precise genetic manipulation of engineered immune cells, making them more universally compatible and thus more accessible for a larger group of cancer patients.
The work is published in the journal Cell.
Overcoming delivery challenges in gene editing
Modern genome editing techniques, including CRISPR systems, hold great potential for treating genetic diseases. However, delivering these molecular tools reliably to their target cells remains a significant challenge.
“Previous viral and non-viral delivery systems such as adeno-associated viruses (AAVs), lipid nanoparticles (LNPs), and other virus-like particles (VLPs), have been valuable but face limitations,” says Dr. Dong-Jiunn Jeffery Truong, last author of the study and group leader at the Institute for Synthetic Biomedicine at Helmholtz Munich.
“Challenges include the increased persistence of gene editors potentially causing immune reactions, or simply their limited efficiency. ENVLPE directly addresses these issues while its modular design maintains compatibility with future gene-editing advancements.”
ENVLPE is based on modified, non-infectious virus-derived shells. These act as carriers for molecular gene editors such as base or prime editors—specialized CRISPR tools that can chemically change single DNA bases in the genome and remove or insert new DNA sequences. ENVLPE’s design solves the logistics challenge of previous methods during the production of the VLPs by hijacking the intracellular transport mechanism so that all components come together at the right time and place.
Prior methods often included partially assembled, non-functional gene editors, reducing delivery effectiveness.
“ENVLPE now not only ensures the packaging of fully assembled gene editors, but also contains an extra molecular shield that protects the most vulnerable part of the editor from degradation during transport,” explains Truong. “This allows the genetic tools to be safely delivered into target cells where the intended DNA edit can take place.”
Restoring vision: Gene editing in action
In close collaboration with a team led by Prof. Krzysztof Palczewski, a professor of ophthalmology at UC Irvine, the scientists tested the ENVLPE system in a mouse model of inherited blindness.
“The mice carry a disabling mutation in the Rpe65 gene, which is essential for producing light-sensitive molecules in the retina, and therefore are fully blind and unresponsive to light,” explains Samuel W. Du, a co-author and MD/Ph.D. candidate at UC Irvine.
After injecting ENVLPE into the subretinal space (the area between the retinal pigment epithelium and photoreceptors) to correct the mutation, the animals began to respond to light stimuli again.
“The extent of restoration was astounding,” says Julian Geilenkeuser, co-first author of the study and a doctoral researcher at the Institute for Synthetic Biomedicine. “It showed us that our particles have real therapeutic potential in a living animal.”
Compared to established systems, ENVLPE achieved significantly better results: A competing system required more than 10 times the dose to achieve similar effects.
“Our goal was to build a tool that is both useful for researchers and suitable for real-world applications,” says Niklas Armbrust, also co-first author and a doctoral researcher at the Institute for Synthetic Biomedicine. “We resolved critical bottlenecks and achieved much more efficient packaging by the delivery agents.”