he FDA has granted Priority Review to Precigen’s Biologics License Application (BLA) for its lead candidate PRGN-2012, the company said—a step forward for a gene therapy that, if approved, would be the first treatment indicated for adults with the rare disease of recurrent respiratory papillomatosis (RRP).
The FDA also set an August 27 target date for deciding on Precigen’s BLA under the Prescription Drug User Fee Act (PDUFA). PRGN-2012—which Precigen has begun to call by its generic name of zopapogene imadenovec—is a gene therapy designed to elicit immune responses directed against cells infected with human papillomavirus (HPV) 6 or HPV 11.
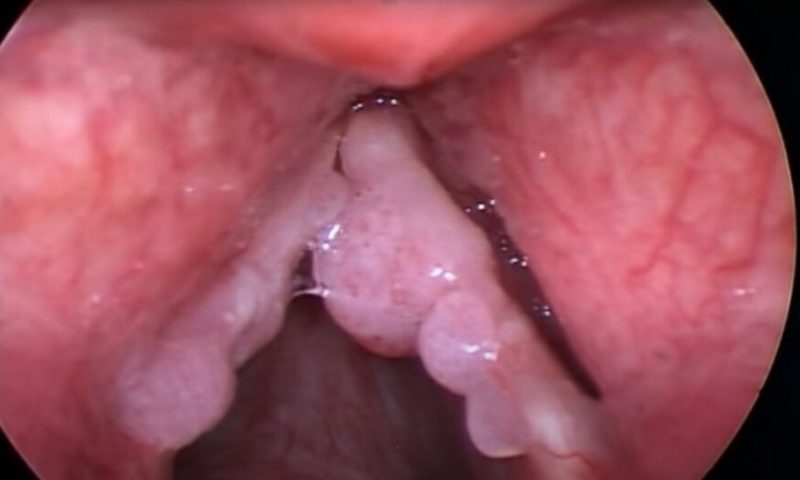
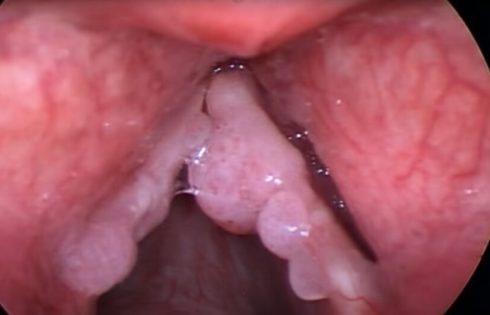
Infection with HPV 6 or HPV 11 causes RRP, a lifelong neoplastic disease of the upper and lower respiratory tracts that can be fatal. According to Precigen, some 27,000 U.S. adults have RRP based on a recently updated internal analysis derived from claims data, and more than 125,000 patients outside the United States have the disease. The standard of care for RRP patients consists of numerous surgeries.
Precigen has projected a “multi-billion-dollar global blockbuster potential” for PRGN-2012. The publicly traded company has not guided investors to a projected list price for the gene therapy, though president and CEO Halen Sabzevari, PhD, told GEN Edge recently that the company has focused on late clinical development and eventual commercialization of PRGN-2012 since the summer.
In July, Precigen announced the appointment of Phil Tennant as chief commercial officer, with initial duties focused on overseeing commercial readiness activities for the potential launch of PRGN-2012.
“We have been basically building our commercial force, and have Phil’s leadership of the commercial effort that he has already assembled, and we feel that we will be ready to launch immediately after the FDA approval,” Sabzevari said.
That effort, she added, will entail Precigen teaming up with partners experienced in commercial activity who will report directly to the company’s commercial force: “It will be completely under our control. We believe that that would be the fastest way for us and also more effective, instead of just building every unit directly from inside.”
Precigen declared the advancement of PRGN02012 its first priority when it reprioritized its clinical portfolio and resources last August, in the process eliminating over 20% of its workforce. Precigen now has about 100 jobs, though the company plans to add some executives in strategic positions, especially in commercial medical affairs, “to address the needs that we have for PRG in 2012 at this moment,” Sabzevari said.
60% surge
Investors appeared to warm up slowly to the news on PRGN-2012, as Precigen shares Tuesday dipped 1% from $1.75 to $1.73, before climbing 6% to $1.84 in early Tuesday trading as of 11:38 am ET. Precigen’s shares have surged 60% over the past six months, climbing from $1.15 on August 26, 2024, as the company has shared a series of positive updates on the clinical development of PRGN-2012 and its other pipeline candidates.
PRGN-2012 was developed through the company’s AdenoVerse® platform, which uses a library of adenovectors for efficient gene delivery of therapeutic effectors, immunomodulators, and vaccine antigens designed to modulate the immune system. Precigen said its AdenoVerse gene therapies have been shown to generate high-level and durable antigen-specific T-cell immune responses, as well as boost these responses via repeat administration.
Precigen’s BLA was supported by positive data from a pivotal Phase I/II trial (NCT04724980) that was presented in June at the 2024 American Society of Clinical Oncology (ASCO) annual meeting, and published last month in The Lancet Respiratory Medicine.
The trial met its primary outcome measure, with 18 of 35 patients (51%) achieving complete response, defined as the percentage of patients who did not require an intervention to control RRP in the 12 months after treatment. Thirty of the 35 patients (86%) experienced a decrease in surgical interventions in the year after PRGN-2012 treatment compared to the year before starting treatment. Even more encouraging: patients treated with PRGN-2012 went from a median of four surgeries pre-treatment to zero post-treatment.
Key secondary endpoints included HPV-specific immune responses, the extent of papilloma growth as measured by Derkay scoring, and quality of life as measured by Vocal Handicap Index-10 (VHI-10).
PRGN-2012 earlier received the FDA’s Breakthrough Therapy and Orphan Drug designations, plus an accelerated approval pathway from the agency, as well as the European Commission’s Orphan Drug Designation.
Also in advanced clinical development is a combination therapy of PRGN-2009, a first-in-class AdenoVerse gene therapy for HPV cancers, plus Merck & Co.’s blockbuster cancer immunotherapy Keytruda® (pembrolizumab). The combination is being studied in a Phase II trial (NCT05996523) in newly diagnosed HPV-associated oropharyngeal squamous cell carcinoma (OPSCC), and another Phase II study (NCT06157151) in recurrent or metastatic cervical cancer. Precigen is conducting both trials under a cooperative research and development agreement (CRADA) with the National Cancer Institute (NCI).
PRGN-2009 is designed to optimize HPV 16/18 antigen design and delivery using a gorilla adenovector with a large payload capacity and the ability for repeat administration. PRGN-2009 uses AdenoVerse and Precigen’s UltraVector® platform, which incorporates advanced DNA construction technologies and computational models to design and assemble genetic components into complex gene expression programs. According to the company, UltraVector-enabled matrices facilitate rapid identification of components that yield desired gene expression.
Enrollment pause
Last August as part of its pipeline reprioritization, Precigen paused enrollment in the cervical cancer Phase II trial at non-NCI sites—including the University of Arkansas for Medical Sciences and the University of Washington, according to the trial’s page on ClinicalTrials.gov. The study’s estimated primary completion date is January 2028, compared with November 2025 for the OPSCC trial.
Precigen’s pipeline also includes three candidates based on its chimeric antigen receptor (CAR) T-cell therapy platform called UltraCAR-T®, all in trials listed as active but not recruiting patients on ClinicalTrials.gov:
• PRGN-3005, an ovarian cancer candidate using Precigen’s non-viral gene delivery system to simultaneously express a CAR optimized to preferentially target the unshed portion of Mucin 16 (MUC16) on tumor cells, membrane-bound interleukin-15 (mbIL15), and a kill switch. PRGN-3005 has been under study in a Phase I trial (NCT03907527).
• PRGN-3006, a candidate for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) simultaneously expressing a CAR targeting CD33, mbIL15, and a kill switch. Last year Precigen said it completed enrollment in a Phase Ib trial in AML (NCT03927261).
• PRGN-3007, a blood tumor and solid tumor candidate simultaneously expressing a CAR targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1), mbIL15, a kill switch, and a novel mechanism for the intrinsic blockade of programmed cell death protein 1 (PD-1) gene expression. PRGN-3007 has been under study in a Phase I trial (NCT05694364). Among diseases under study in the trial are hematologic chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), and solid tumor triple-negative breast cancer (TNBC) malignancies.
Precigen has said it is seeking partnerships to pursue the development of PRGN-3008, a CD19-targeting pipeline candidate developed to treat forms of cancer and autoimmune diseases. “We have been basically discussing some of the inbound requests that we have had for partnerships. We think that will be the best way for us to advance the Ultra-CAR-T platform at this point,” Sabzevari said.
What skills are Precigen seeking in a partner for PRGN-3008?
“Definitely the importance of this is to have the financial resources” since Precigen has focused its finances on advancing and eventually commercializing PRGN-2012, Sabzevari said. “The other aspects is, of course, the knowledge in the cell therapy, advancement of that, and also it will be very helpful to have commercial teams that are on the ground.”
UltraCAR-T is a chimeric antigen receptor T-cell therapy approach designed to differentiate from the CAR-T platforms of competitors by offering increased patient access through rapid manufacturing, lower manufacturing-related costs, and advanced technologies for precise tumor targeting and control of the immune system to achieve improved outcomes. UltraCAR-T cells use the non-viral Sleeping Beauty system, which has been optimized using Precigen’s UltraVector DNA construction platform to deliver a large multigenic payload at high efficiency.