In a development that sounds fit for a horror film, scientists at Stanford University have transplanted human brain cells into rats. These human-rat brain hybrids could be used to study conditions like autism and schizophrenia, potentially marking a major turning point in scientists’ ability to develop effective treatments.
“The human brain certainly has not been very accessible, which precluded the progress we’ve been making in understanding the biology of these conditions,” Professor Sergiu Pasca, co-corresponding author and director of Stanford Brain Organogenesis, told reporters in an online press briefing. “That’s why building human models that are noninvasive of the human brain is one of the most promising avenues in trying to tackle these conditions.”
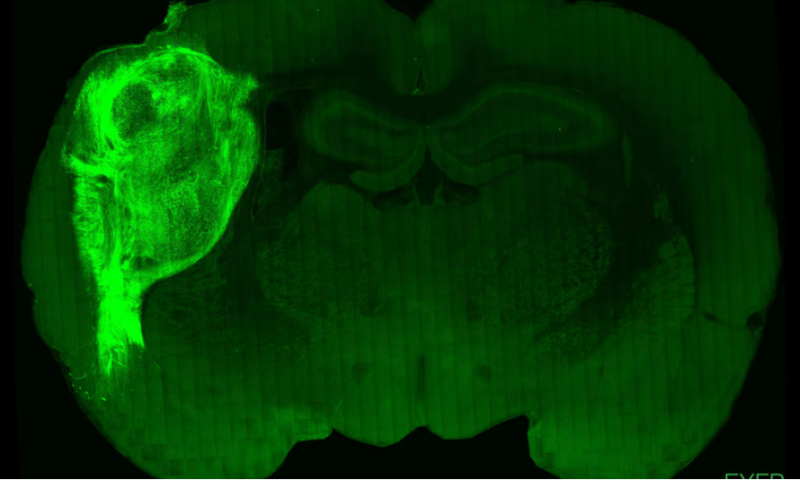
In a study published Sept. 12 in Nature, the scientists described how they grew stem cells into tiny 3D structures called organoids, which they transplanted into newborn rats’ somatosensory cortices—the part of the brain that receives signals from their sensory organs, including their whiskers, before they’re sent elsewhere in the brain for interpretation. There, the structures melded with the rats’ growing brains, sprouting new neurons. They were fully integrated a little more than six months later, complete with blood vessels and short- and long-range connections to other cells.
With the cells wired into the rat brains, the scientists could study them using the standard tools of the neuroscience trade like electrodes and calcium imaging. First, to see whether the “human” parts of the brain would respond to external stimuli, the scientists ruffled the rats’ whiskers on the opposite side of where the cells were implanted. The stimulation caused the cells to fire, indicating that they were functional.
Next, the scientists sought to find out whether the cells could influence the rats’ behavior. Prior to the study, they genetically altered a set of the organoids to be stimulated when they were hit with blue light from a fiber-optic cable, which was later implanted in the rats’ brains. They then alternately changed the color of the light from blue or red. If the rats licked a spout when the light was blue, they received water. By the end of the two-week study period, the rats licked the spout more frequently when the light was blue, indicating that the human cells were activating rat neurons to drive reward-seeking behavior.
While the results are intriguing, there are some limitations. For instance, the scientists noted, differences between rodents and humans will make it challenging to truly replicate human brain circuits in rat brains. Furthermore, the implanted cells do not “laminate,” or stack, the way organically grown cells do. That could alter their ability to connect to each other.
Limitations aside, it’s difficult to say whether the hybrid model will become a new standard for research, and concerns have been raised about the ethics of implanting human neurons in animals. (Though the researchers noted that the rats showed no more signs of distress or pain than rats with normal brains.) At this point, it’s probably still too expensive and complex to become commonplace, Harvard molecular biologist Paola Arlotta, Ph.D., told Nature.
The scientists are moving forward with their research, expanding into new cells types like microglia and endothelial cells. Eventually, they hope to use their model to develop new drugs for neuropsychiatric disorders.