Flying high from a second quarter that saw sales jump more than 40%, NuVasive is setting itself up for an even bigger second half of the year, thanks to a newly bestowed FDA clearance for its Pulse spine surgery platform.
The U.S. clearance rolled in as NuVasive began rolling out the device in other regions around the globe, backed by a CE mark the technology received in June approving it for commercialization and clinical use in Europe.
With the FDA’s blessing, that rollout will now be extended to the U.S.
Both the U.S. and European green lights come several years after the Pulse platform received its first regulatory clearances in 2018. Rather than immediately launching the system at the time, however, NuVasive opted to continue tweaking and updating its technology, then re-submitted its applications and began rolling out the platform only once it had reached its full potential.
“Surgeons are now able to seamlessly work with various technologies to address more clinical challenges in surgery from a single platform—something they could not do before Pulse,” said CEO J. Christopher Barry.
“This is the culmination of years of research and development to deliver a platform that helps improve clinical, financial and operational outcomes.”
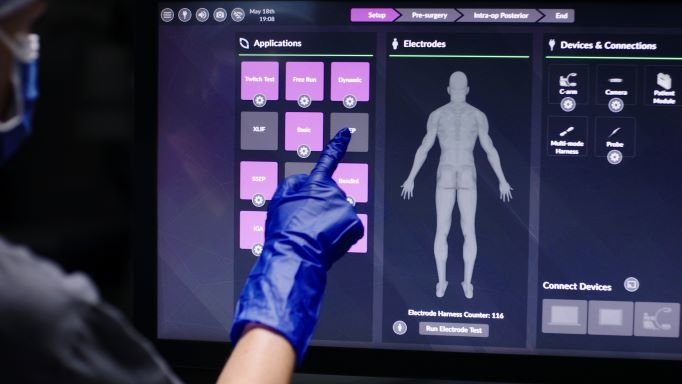
Pulse combines a variety of tools and technologies into a single platform to streamline all types of spinal surgery. For example, surgeons can look to for guidance through a procedure, as Pulse includes tools to improve screw placement accuracy, spinal alignment and neuromonitoring. It also connects to NuVasive’s Bendini spinal rod bending tool, which provides automated, patient-specific instructions to assist with manual rod bending.
The system not only integrates with a variety of imaging platforms like the Siemens Cios Spin mobile C-arm but is also equipped with technology that can further boost the quality of images collected by those platforms, reducing the level of radiation needed to acquire high-quality data during an operation.
The entire surgical team can access NuVasive’s all-in-one platform wirelessly before, during and after a procedure. Furthermore, the system will be continuously updated to accommodate future developments in robotics and other smart tools.
The regulatory nods and global commercial launch of the Pulse system will undoubtedly keep NuVasive riding the post-pandemic surge that saw it clock just under $300 million in sales for the second quarter of the year, about a 43% bump from the same period in 2020.
That windfall arrived even as the San Diego-based company temporarily halted shipping of another of its product lines, the MAGEC spinal curve-correcting magnetic rod systems. The April hold came after NuVasive spent the previous year updating the devices to reduce an issue in which their endcaps came loose, with a risk of exposing patients to internal components that hadn’t yet been fully evaluated for biocompatibility.
Shipping of the devices resumed earlier this month—with support from the FDA—as NuVasive discontinued all versions of the MAGEC system except the most recently updated edition of the Model X and confirmed its continued efforts alongside the FDA to evaluate the safety of the inner parts of the device.