Influenza B Protein Structure – MIT Chemists Reveal the Protein Structure
To treat influenza B infections, the findings might help researchers design drugs.
The structure of a key influenza protein has been discovered by a team of MIT chemists. This finding could help scientists design drugs that prevent the virus from spreading by blocking the protein.
A proton channel that controls acidity within the virus, the protein called BM2 helps the virus in releasing its genetic material inside cells that are infected.
The senior author of the study and an MIT professor of chemistry, Mei Hong said, “You can have a way to inhibit influenza infection if you can block this protein channel. To start designing small molecules that can block the protein, medicinal chemists and pharmaceutical scientists need the atomic-resolution structure for this protein and this is exactly what is found in our study.” Venkata Mandala, MIT graduate student is the lead author of the paper. Other contributors include an associate professor of chemistry Bradley Pentelute and graduate students Alexander Shcherbakov and Alexander Loftis.
In the journal Nature Structural and Molecular Biology, this study is published.
Atomic-scale resolution
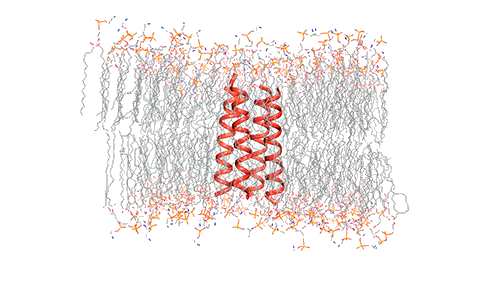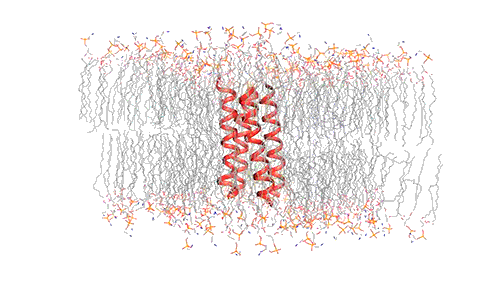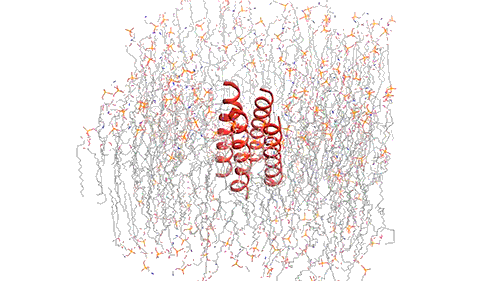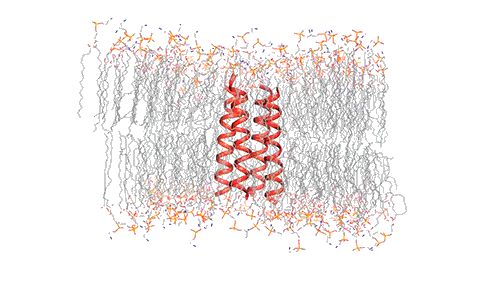
Influenza virus has three class — A, B, and C and each of the viruses produce the M2 protein in different versions. Through the virus’s outer member, called the lipid envelope, this M2 ion, an ion channel, carriers protons through it. Most of the structural studies of the M2 protein until now have focused on the M2 version found in influenza A, which is the most common form usually. However, the researchers in this study focused on the M2 version found in influenza B viruses.
According to infections reported to the U.S. Centers for Disease Control since last September, influenza B has been dominant usually, accounting for 67 percent of all flu cases, in contrast to the previous patterns of seasonal flu infections.
Hong and her colleagues set out to study the structural differences these proteins might have, and how their functions are influenced by those differences as the A and B versions of M2 vary significantly in their amino acid sequences. The AM2 channel allows protons to flow into the viral envelope and the BM2 channel can allow protons to flow in either direction, this is one key difference between them.
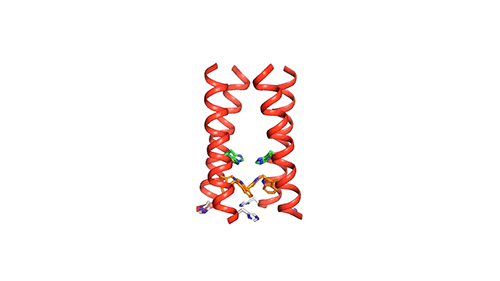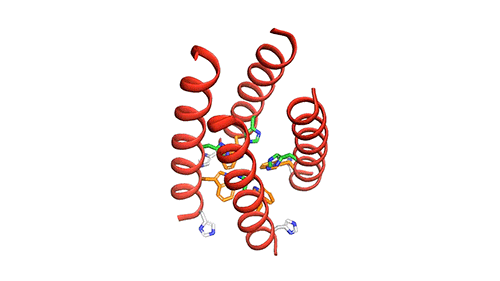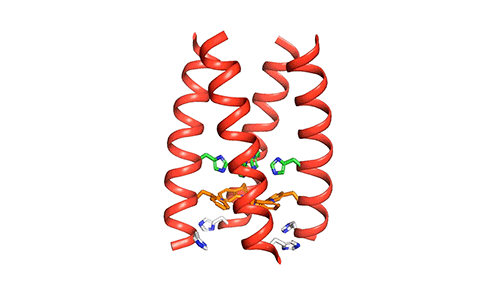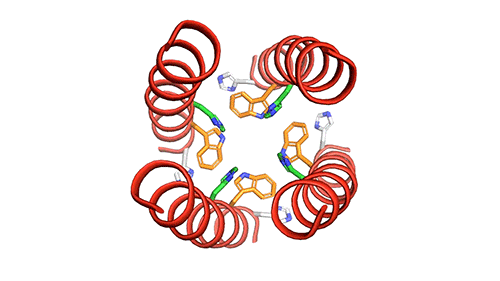
The researchers used nuclear magnetic resonance (NMR) spectroscopy to analyze the structure with atomic-scale resolution after embedding the BM2 into a lipid bilayer, similar to a cell membrane. Due to the difficulty of studying proteins embedded within membranes, very few ion channels have been studied at such high resolution.
There are four helices which makes up the M2 channel. These four helices run through the membrane parallel to each other. Hong identified that depending on the pH of the environment outside the viral envelope, the alignment of these helices slightly changes. The helices are titled by about 14 degrees when the pH is high and the channel is closed. The channel opens like a pair of scissors as the helices increase their tilt to about 20 degrees when the pH goes down. There is greater space created between the helices and more water is allowed to get into the channel by this scissoring motion.
The MIT team believes that the presence of an extra histidine at the virion-facing end of the BM2 channel, unlike the AM2 channel is the reason why the protons flow in either direction through the BM2 channel. The researchers say that to determine what kind of advantage this may provide for influenza B viruses, more studies and research are needed.
Blocking the channel
The chemists can now try to come up with ways to block the BM2 channel as they know the structure of both the open and closed states of this channel at atomic resolution. For this type of drug development, there is a precedent: Rimantadine and amantadine, both used in treating influenza A, work by cutting off the flow of protons in the AM2 channel by wedging themselves into the channel pore. However, there is no effect on the BM2 channel by these drugs.
Another function of BM2, which generates curvature in lipid membranes in order to allow progeny viruses to be released from cells, is been investigated by Hong’s research group. In previous studies, it was seen that a structure called a beta-sheet is formed by a portion of the protein that sticks out from the membrane and that this plays a role in inducing the membrane to curve inward.
The National Institutes of Health has funded this research.

