Six months after netting the FDA’s green light for its groundbreaking portable MRI machine, Hyperfine Research has secured a second clearance for a newer generation of the device.
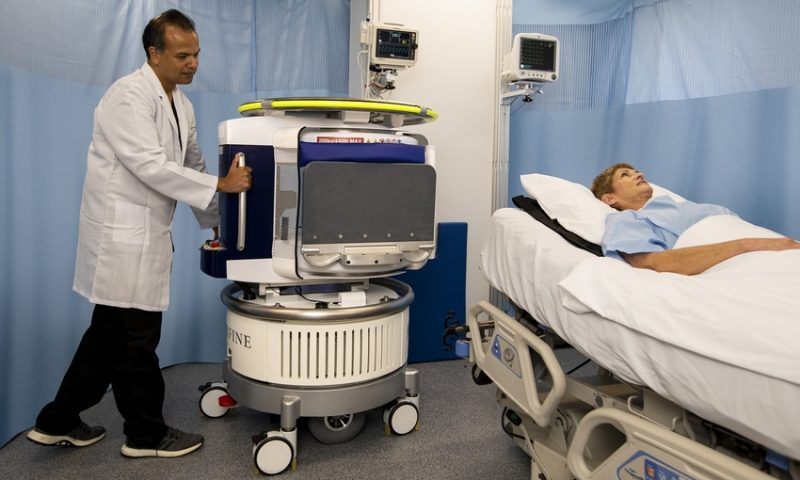
The Swoop system brings point-of-care imaging to the patient’s bedside, with a scanner-on-wheels that plugs into a standard electric wall outlet and fits in an elevator.
Designed to scan a person’s head and brain, the latest clearance expands the system’s use to young toddlers, infants and neonates, after it was initially available to patients ages 2 and older. The upgraded version also includes enhanced imaging and software updates in response to user feedback, Hyperfine said.
“Six years ago, we had a crazy vision to create a new product category for imaging: an affordable point-of-care MRI system,” founder and chairman Jonathan Rothberg said, adding that the new clearance helps deliver on the company’s “mission to democratize healthcare across clinical settings and geographies.”
Hyperfine’s system is designed to complement traditional hospital MRIs, which typically require strengthened rooms and heavier infrastructure to operate. The portable system can help relieve the logistical burdens and health risks of transporting a patient receiving intensive care to an imaging suite, which can take additional time and create backlogs of less-urgent cases.
In addition, the company says that new users can be trained how to safely operate and relocate the system in about 30 minutes, with its MRI scans being run off a wireless tablet.
The device produces images using a combination of low-power radio waves and permanent magnets, which require no additional power or cooling. Hyperfine says its system could be used in a variety of settings such as neurology ICUs, emergency departments and outpatient surgery centers.