As Altoida’s Alzheimer’s disease screening platform inches closer to FDA clearance, the company is bulking up its clinical team.
All of its medical and clinical affairs will now be led by Antonella Santuccione Chadha, M.D., Altoida’s inaugural chief medical officer. The newly created role will report directly to the chief executive, Travis Bond.
“Dr. Chadha is a true maverick in the field of neuroscience and a pioneer in utilizing precision technologies to improve the way we measure brain health and diagnose neurological diseases. She has led high-performing teams in the private, public and nonprofit sectors and has a proven track record of success in R&D, clinical and regulatory affairs,” Bond said in a statement.
“She will be an invaluable asset to Altoida and our leadership team as we expand our platform and advance our deep pipeline of novel digital biomarkers for Alzheimer’s and other neurological and neuropsychiatric disorders,” he continued.
Chadha joins the Washington, D.C.-based startup after spending decades honing her expertise in Alzheimer’s and other neurological conditions as a practicing physician and neuroscientist.
She spent several years at Roche, including stints as its senior medical director focusing on Alzheimer’s and then as the medical director for the company’s partnerships pertaining to the disease. After that—and up until April of this year—she served as the head of stakeholder engagement for Biogen’s Alzheimer’s-related projects. Biogen, of course, won the first Alzheimer’s approval in decades last year, but the drug has been criticized, and the Centers for Medicare & Medicaid Services refused to cover it for routine use.
Outside of those roles, Chadha co-founded the Women’s Brain Project in 2016. The nonprofit—which she also leads as pro bono CEO—studies how diseases can differ based on sex and gender and how those factors affect development of drugs, diagnostics and other new medical technologies.
“I am thrilled to join Altoida at such a dynamic time … The company’s proprietary AI and augmented reality technology will be transformative in the early and accurate detection of Alzheimer’s and across the entire neurological disease drug development life cycle,” Chadha said.
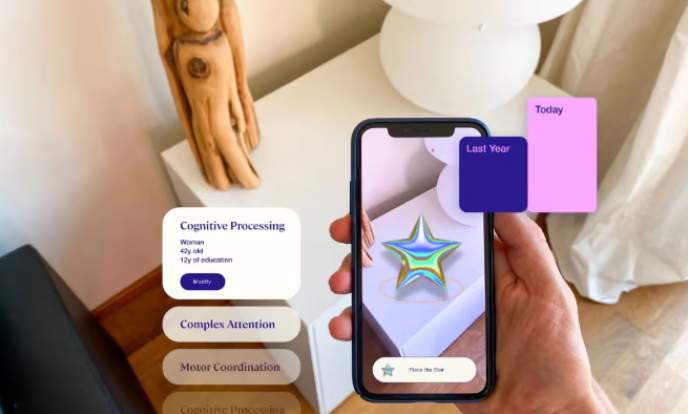
Altoida is developing a software platform that uses artificial intelligence and augmented reality technology to evaluate an individual’s brain health. Its smartphone app offers a set of game-ified tests, the results of which are used to calculate the likelihood that a case of mild cognitive impairment will escalate into Alzheimer’s within a year.
“I look forward to working with Travis and the Altoida team to reduce the time and cost to diagnose complex and devastating conditions like Alzheimer’s, by bringing innovative, precise digital measurements and diagnostics to patients in need,” Chadha said in the release.
Last August, the company said its technology received the FDA’s breakthrough designation, which may help smooth the process of applying for regulatory clearance once Altoida wraps up clinical trials of the software.
Altoida’s app-based screening tool is one of only a handful currently in development to deliver a digital alternative to traditional blood tests and brain scans for Alzheimer’s diagnosis. In 2019, Apple, Eli Lilly and Evidation Health published early results from a study showing that readings from iPhones, Apple Watches, iPads and Apple’s Beddit sleep monitor could be used to detect early signs of cognitive decline.
And just a few weeks ago, researchers from the University of California, San Diego presented a paper describing an app that uses a smartphone’s built-in facial recognition and selfie cameras to measure changes in the size of a person’s pupil, which may be linked to neurological function.